Cancer treatment
This page is a Wikiversity content development project for topic of Cancer Treatment.
When ready, please migrate all learning materials to pages in the main namespace (no prefix in the page names).
Cancer Treatment
[edit | edit source]Chemotherapy
[edit | edit source]Colorectal Cancer
[edit | edit source]FDA approved drugs
[edit | edit source]Fluorouracil
[edit | edit source]
Fluorouracil (5-FU) is a drug that is used in the treatment of cancer. It belongs to the family of drugs called antimetabolites. It is a pyrimidine analog.
The chemotherapy agent 5-FU (fluorouracil), which has been in use against cancer for about 40 years, acts in several ways, but principally as a thymidylate synthase inhibitor, interrupting the action of an enzyme which is a critical factor in the synthesis of pyrimidine-which is important in DNA replication.
Some of its principal use is in colorectal cancer and pancreatic cancer, in which it has been the established form of chemotherapy for decades (platinum-containing drugs are a recent addition).
As a pyrimidine analogue, it is transformed inside the cell into different cytotoxic metabolites which are then incorporated into DNA and RNA, finally inducing cell cycle arrest and apoptosis by inhibiting the cell's ability to synthesize DNA. It is an S-phase specific drug and only active during certain cell cycles.
Capecitabine is a prodrug that is converted into 5-FU in the tissues. It can be administered orally.
From: Fluorouracil
Uracil can be used for drug delivery and as a pharmaceutical. When elemental fluorine is reacted with uracil, 5-fluorouracil is produced. 5-Fluorouracil is an anticancer drug (antimetabolite) used to masquerade as uracil during the nucleic acid replication process. The drug molecule also fools the enzymes that help in this process to incorporate this compound in the replication and not uracil, this causes the biological polymer (cancer) not to continue synthesizing.
From: Uracil
The backbone of treatment for colorectal cancer is fluorouracil, a fluorinated pyrimidine, which is thought to act primarily by inhibiting thymidylate synthase, the rate-limiting enzyme in pyrimidine nucleotide synthesis. Fluorouracil is usually administered with leucovorin, a reduced folate, which stabilizes the binding of fluorouracil to thymidylate synthase, thereby enhancing the inhibition of DNA synthesis. In patients with advanced colorectal cancer, treatment with fluorouracil and leucovorin reduces tumor size by 50 percent or more in approximately 20 percent of patients (the "objective-response rate") and prolongs median survival from approximately 6 months (without treatment) to about 11 months.
From: Systemic Therapy for Colorectal Cancer
Thymidylate synthase (TS) catalyzes the transfer of a methyl group from methylenetetrahydrofolate (CH2H4PteGlu) to dUMP forming TMP. Inhibition of TS results in apoptotic cell death due to intracellular thymidine depletion. Since cancer cells undergo rapid multiplication, they are much more sensitive to thymidine depletion and TS is the target of several anticancer agents used in colon, neck, and breast chemotherapy.
From: Thymidylate synthase
Antimetabolite drugs work by inhibiting essential biosynthetic processes, or by being incorporated into macromolecules, such as DNA and RNA, and inhibiting their normal function. The fluoropyrimidine 5-fluorouracil (5-FU) does both. Fluoropyrimidines were developed in the 1950s following the observation that rat hepatomas used the pyrimidine uracil — one of the four bases found in RNA — more rapidly than normal tissues, indicating that uracil metabolism was a potential target for antimetabolite chemotherapy1. The mechanism of cytotoxicity of 5-FU has been ascribed to the misincorporation of fluoronucleotides into RNA and DNA and to the inhibition of the nucleotide synthetic enzyme thymidylate synthase (TS).
5-FU is widely used in the treatment of a range of cancers, including colorectal and breast cancers, and cancers of the aerodigestive tract. Although 5-FU in combination with other chemotherapeutic agents improves response rates and survival in breast and head and neck cancers, it is in colorectal cancer that 5-FU has had the greatest impact. 5-FU-based chemotherapy improves overall and disease-free survival of patients with resected stage III colorectal cancer. Nonetheless, response rates for 5-FU-based chemotherapy as a first-line treatment for advanced colorectal cancer are only 10–15% (REF. 3). The combination of 5-FU with newer chemotherapies such as Irinotecan and Oxaliplatin has improved the response rates for advanced colorectal cancer to 40–50%. However, despite these improvements, new therapeutic strategies are urgently needed.
Understanding the mechanisms by which 5-FU causes cell death and by which tumours become resistant to 5-FU is an essential step towards predicting or overcoming that resistance. So, what do we know about the mechanism of action of 5-FU and what strategies have been used to enhance its activity?
...5-FU is an analogue of uracil with a fluorine atom at the C-5 position in place of hydrogen. It rapidly enters the cell using the same facilitated transport mechanism as uracil6. 5-FU is converted intracellularly to several active metabolites: fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP) (FIG. 1) — these active metabolites disrupt RNA synthesis and the action of TS. The rate-limiting enzyme in 5-FU catabolism is dihydropyrimidine dehydrogenase (DPD), which converts 5-FU to dihydrofluorouracil (DHFU).More than 80% of administered 5-FU is normally catabolized primarily in the liver, where DPD is abundantly expressed.
From: 5-Fluorouracil: Mechanisms of Action and Clinical Strategies
Capecitabine (Xeloda)
[edit | edit source]
Capecitabine (INN) (IPA: [keɪpˈsaɪtəbin]) is an orally-administered chemotherapeutic agent used in the treatment of metastatic breast and colorectal cancers. Capecitabine is a prodrug, that is enzymatically converted to 5-fluorouracil in the tumor by the tumor-specific enzyme PynPase, where it inhibits DNA synthesis and slows growth of tumor tissue. The activation of capecitabine follows a pathway with three enzymatic steps and two intermediary metabolites, 5'-deoxy-5-fluorocytidine (5'-DFCR) and 5'-deoxy-5-fluorouridine (5'-DFUR), to form 5-fluorouracil. Capecitabine is marketed under the trade name Xeloda (Roche).
Capecitabine is FDA-approved for:
- Adjuvant Stage III Dukes'C Colon Cancer - used as first-line monotherapy.
- Metastatic Colorectal Cancer - used as first-line monotherapy, if appropriate.
- Metastatic Breast Cancer - used in combination with docetaxel, after failure of anthracycline-based treatment. Also as monotherapy, if the patient has failed paclitaxel-based treatment, and if anthracycline-based treatment has either failed or cannot be continued for other reasons (i.e., the patient has already received the maximum lifetime dose of an anthracycline).
From: Capecitabine
Irinotecan (Camptosar)
[edit | edit source]
Irinotecan is a chemotherapy agent that is a topoisomerase 1 inhibitor.
Its main use is in colon cancer, particularly in combination with other chemotherapy agents. This includes the regimen FOLFIRI which consists of infusional 5-fluorouracil, leucovorin, and irinotecan.
Irinotecan is marketed by Pfizer as Camptosar®. It is also known as CPT-11.
Irinotecan is activated by hydrolysis to SN-38, an inhibitor of topoisomerase I. This is then inactivated by glucuronidation by uridine diphosphate glucoronosyltransferase 1A1 (UGT1A1). Eventually, this process inhibits DNA replication and transcription.
From: Irinotecan
Oxaliplatin (Eloxatin)
[edit | edit source]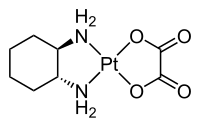
Oxaliplatin is a platinum-based chemotherapy drug in the same family as cisplatin and carboplatin. It is typically administered in combination with fluorouracil and leucovorin in a combination known as FOLFOX for the treatment of colorectal cancer. Compared to cisplatin the two amine groups are replaced by cyclohexyldiamine for improved antitumour activity. The chlorine ligands are replaced by the oxalato bidentate derived from oxalic acid in order to improve water solubility.
Oxaliplatin is marketed by Sanofi-Aventis under the trademark Eloxatin®.
In vivo studies showed oxaliplatin has anti-tumor activity against colon carcinoma through its (non-targeted) cytotoxic effects. Median patient survival is approximately 5 months greater compared to the previous standard treatment.
From: Oxaliplatin
Cetuximab (Erbitux)
[edit | edit source]Cetuximab (Erbitux®) is a chimeric monoclonal antibody given by intravenous injection for treatment of metastatic colorectal cancer and head and neck cancer.
Cetuximab is distributed inside the United States by ImClone Systems and Bristol-Myers Squibb, while it is distributed outside North America by Merck KGaA. It faces stiff competition from bevacizumab (Avastin), made by Genentech, and potential competition from panitumumab, currently under development by Amgen and Abgenix.
Cetuximab is believed to operate by binding to the extracellular domain of the epidermal growth factor receptor (EGFR) of cancer cells, preventing ligand binding and activation of the receptor. This blocks the downstream signaling of EGFR resulting in impaired cell growth and proliferation.
Cetuximab is used in metastatic colon cancer and is given concurrently with the chemotherapy drug irinotecan (Camptosar®), a form of chemotherapy that blocks the effect of DNA topoisomerase I, resulting in fatal damage to the DNA of affected cells. While there is a medical laboratory test to detect if a cancer tumor overexpresses epidermal growth factor receptor(EGFR) on its cells surface, this overexpression has recently been shown to not have any bearing on whether a patient will respond to Cetuximab or not. Whether this is because the current tests are just not sensitive enough to detect EGFR overexpression or because EGFR overexpression is not linked to the drugs effectiveness has not been established. Cetuximab was approved by the FDA in March 2006 after the publication of research performed by Dr J. Bonner [1] for use in combination with radiation therapy for treating squamous cell carcinoma of the head and neck (SCCHN) or as a single agent in patients who have had prior platinum-based therapy.
The probability of successfully responding to Cetuximab therapy is linked to the incidence of acne like rash, one of the drugs side effects. The worse the rash that develops for the patient the higher the response rate.
From: Cetuximab
Bevacizumab (Avastin)
[edit | edit source]Bevacizumab (trade name Avastin®) drug used in treatment of cancer that targets the angiogenesis pathway.
It is used in combination with standard chemotherapy drugs in patients with metastatic colorectal cancer. The U.S. Food and Drug Administration approved bevacizumab for use in colon cancer 2004. The medicine was developed by Genentech and is marketed, in the United States by Genentech and elsewhere by Roche (Genentech's parent company), under the brand name Avastin.
Bevacizumab is a humanized monoclonal antibody, and was the first commercially available angiogenesis inhibitor. It stops tumor growth by preventing the formation of new blood vessels by targeting and inhibiting the function of a natural protein called vascular endothelial growth factor (VEGF) that stimulates new blood vessel formation.
The drug was first developed as a genetically engineered version of a mouse antibody that contains both human and mouse components. Genentech is able to produce the antibody in production-scale quantities.
Bevacizumab was approved by the Food and Drug Administration (FDA) in February 2004 for use in colorectal cancer when used with standard chemotherapy treatment. It was approved by the EMEA in January 2005 for use in colorectal cancer. Israel has also approved the use of bevacizumab.
Bevacizumab is usually given intravenously through the arm every 14 days. In colon cancer, it is given in combination with the chemotherapy drug 5-FU (5-fluorouracil), leucovorin, and oxaliplatin or irinotecan.
Bevacizumab has also demonstrated activity in renal cell cancer and ovarian cancer when used as a single agent, and in lung cancer and breast cancer when combined with chemotherapy.
From: Bevacizumab
Protease
[edit | edit source]High levels of proteolytic enzymes are associated with many tumors. This may be a result of adaptation to rapid cell cycling; removal of unnecessary regulatory proteins; and for secretion to sustain invasion, metastasis formation, and angiogenesis. Proteolytic enzymes represent an attractive target of antitumor imaging strategies and potentially antitumor prodrug activation therapy.