Vanadium tetrafluoride
Appearance
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
vanadium tetrafluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.030.143 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | UN2923 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| F4V | |||
| Molar mass | 126.9351 g·mol−1 | ||
| Appearance | Lime green powder, hygroscopic[1] | ||
| Odor | Odorless | ||
| Density | 3.15 g/cm3 (20 °C)[1] 2.975 g/cm3 (23 °C)[2] | ||
| Melting point | 325 °C (617 °F; 598 K) at 760 mmHg decomposes[1] | ||
| Boiling point | Sublimes[1] | ||
| Very soluble[1] | |||
| Solubility | Soluble in acetone, acetic acid Very slightly soluble in SO2Cl2, alcohols, CHCl3[2] | ||
| Structure | |||
| Monoclinic, mP10 | |||
| P21/c, No. 14 | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
126 J/mol·K[3] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−1412 kJ/mol[3] | ||
Gibbs free energy (ΔfG⦵)
|
−1312 kJ/mol[3] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Eye hazards
|
Causes serious damage | ||
Skin hazards
|
Causes burns | ||
| GHS labelling:[4] | |||
 
| |||
| Danger | |||
| H300, H314, H330 | |||
| P260, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P320, P330, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Vanadium(IV) fluoride (VF4) is an inorganic compound of vanadium and fluorine. It is paramagnetic yellow-brown solid that is very hygroscopic.[2] Unlike the corresponding vanadium tetrachloride, the tetrafluoride is not volatile because it adopts a polymeric structure.[5] It decomposes before melting.
Preparation and reactions
[edit]VF4 can be prepared by treating VCl4 with HF:
- VCl4 + 4 HF → VF4 + 4 HCl
It was first prepared in this way.[6]
It decomposes at 325 °C, undergoing disproportionation to the tri- and pentafluorides:[2]
- 2 VF4 → VF3 + VF5
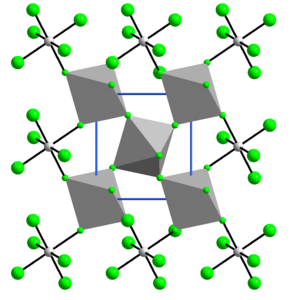
Structure
[edit]The structure of VF4 is related to that of SnF4. Each vanadium centre is octahedral, surrounded by six fluoride ligands. Four of the fluoride centers bridge to adjacent vanadium centres.[7]
References
[edit]- ^ a b c d e Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ a b c d Kwasnik, W. (1963). Brauer, Georg (ed.). Handbook of Preparative Inorganic Chemistry (UK ed.). London: Academic Press. pp. 252–253.
- ^ a b c Anatolievich, Kiper Ruslan. "vanadium(IV) fluoride". chemister.ru. Retrieved 2014-06-25.
- ^ "Vanadium(IV) fluoride, 95%". alfa.com. Alfa Aesar. Retrieved 2014-06-25.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, p. 716, ISBN 0-471-19957-5
- ^ Otto Ruff, Herbert Lickfett "Vanadinfluoride" Chemische Berichte 1911, vol. 44, pages 2539–2549. doi:10.1002/cber.19110440379
- ^ Becker S., Muller B. G. Vanadium Tetrafluoride, Angew. Chem. Intnl. Ed. Engl. 1990, vol. 29, page 406