Eugenin
Appearance
| |
| Names | |
|---|---|
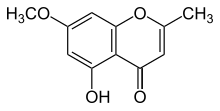
| Preferred IUPAC name
5-Hydroxy-7-methoxy-2-methyl-4H-1-benzopyran-4-one | |
| Other names
5-Hydroxy-7-methoxy-2-methylchromone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H10O4 | |
| Molar mass | 206.197 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Eugenin is a chromone derivative, a phenolic compound found in cloves. It is also one of the compounds responsible for bitterness in carrots.[1]
Derivatives
[edit]- 6-Hydroxymethyleugenin can be isolated from the fungal species Chaetomium minutum.[2]
References
[edit]- ^ Czepa, A; Hofmann, T (2003). "Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree". Journal of Agricultural and Food Chemistry. 51 (13): 3865–73. doi:10.1021/jf034085+. PMID 12797757.
- ^ Hauser, D.; Zardin, Therese (1972). "Isolation of 6-hydroxymethyl-eugenin from Chaetomium minutum". Experientia. 28 (9): 1114–5. doi:10.1007/BF01918708. PMID 4579107. S2CID 7551396.