Organothiophosphates or organophosphorothioates are a subclass of organophosphorus compounds and of thiophosphate compounds. They are the organic compounds that contain a phosphate group in which one or more oxygen atoms is substituted by sulfur. Many are used as pesticides, some have medical applications, and some are used as oil additives.[1] They generally have the chemical formula (RO)3PS, [(RO)2P(S)O]−, R(RO)2PS, etc.
-
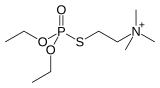
Echothiophate used for treatment of glaucoma
-
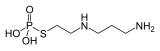
Amifostine, which is used in cancer chemotherapy
-
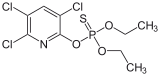
Chlorpyrifos, a common insecticide
-
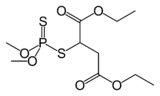
Malathion, a common insecticide
-
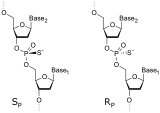
Phosphorothioates are the basis for antisense therapies
Oligonucleotide phosphorothioates (OPS) are modified oligonucleotides where one of the oxygen atoms in the phosphate moiety is replaced by sulfur. These compounds are the basis of antisense therapy, e.g., the drugs fomivirsen (Vitravene), oblimersen, alicaforsen, and mipomersen (Kynamro).[2]
Further examples of these include:
Variants with P=S double bonds were developed as insecticides because of their reduced mammalian toxicity. The phosphorothioate P=S bond is converted to the toxic P=O bond in the target insect. Similar oxidative conversion in mammals is slower, conferring lower toxicity in mammals.
Structure, synthesis, and reactions
editGenerally these compounds feature tetrahedral phosphorus(V) centers. Classically, thiophosphates would include a P=S double bond as illustrated by malathion. The terminology is used loosely and thiophosphates include P-S single bonds as illustrated by the drug amifostine. P–S single bonds can be generated through a variety of approaches, starting from thiols, disulfides, sulfinic acids as sulfur sources and various P(III) and P(V) coupling partners.[3] PS–C bonds can also be formed through many comparable approaches, usually by alkylating a free phosphorus-thioate anion or thioic acid.[4]
They are conceptually derived from the inorganic thiophosphates (PO4−xS3−
x). In fact, many are prepared via the intermediacy diorganodithiophosphoric acids, which are prepared by treating phosphorus pentasulfide with alcohols:[1]
- P2S5 + 4 ROH → 2 (RO)2PS2H + H2S
Dimethyl dithiophosphoric acid and diethyl dithiophosphoric acid are obtained in this way. The former is a precursor to malathion.
In the Michalski reaction, a thiophosphate reacts with chlorine gas to give a diorganylphosphorylsulfenyl chloride:[5]
- (RO)3PS + Cl2 → (RO)2P(O)SCl + RCl
References
edit- ^ a b Svara, Jürgen; Weferling, Norbert; Hofmann, Thomas (2006). "Phosphorus Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a19_545.pub2. ISBN 3527306730.
- ^ Kurreck, Jens (2003). "Antisense technologies. Improvement through novel chemical modifications". European Journal of Biochemistry. 270 (8): 1628–1644. doi:10.1046/j.1432-1033.2003.03555.x. PMID 12694176.
- ^ Jones, David J.; O'Leary, Eileen M.; O'Sullivan, Timothy P. (2020-07-29). "Modern Synthetic Approaches to Phosphorus-Sulfur Bond Formation in Organophosphorus Compounds". Advanced Synthesis & Catalysis. 362 (14): 2801–2846. doi:10.1002/adsc.202000458. hdl:10468/10373. ISSN 1615-4150. S2CID 225475935.
- ^ Jones, David J.; O'Leary, Eileen M.; O'Sullivan, Timothy P. (2018-12-05). "Synthesis and application of phosphonothioates, phosphonodithioates, phosphorothioates, phosphinothioates and related compounds". Tetrahedron Letters. 59 (49): 4279–4292. doi:10.1016/j.tetlet.2018.10.058. hdl:10468/7209. ISSN 0040-4039. S2CID 105852959.
- ^ Almasi, Lucreţia (1971). "The Sulfur–Phosphorus Bond". In Senning, Alexander (ed.). Sulfur in Organic and Inorganic Chemistry. Vol. 1. New York: Marcel Dekker. p. 73. ISBN 0-8247-1615-9. LCCN 70-154612.
External links
edit- Organothiophosphorus+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)