Monatepil is a calcium channel blocker and α1-adrenergic receptor antagonist used as an antihypertensive.[1]
| |
| Names | |
|---|---|
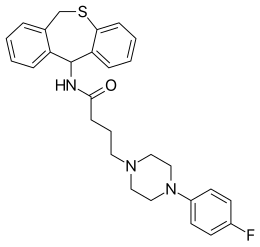
| Preferred IUPAC name
N-(6,11-Dihydrodibenzo[b,e]thiepin-11-yl)-4-[4-(4-fluorophenyl)piperazin-1-yl]butanamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H30FN3OS | |
| Molar mass | 475.63 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editThe synthesis of monatepil was first disclosed in patents filed by Dainippon Pharmaceutical.[2]
The amino group of the dihydrodibenzothiepin (1) is first reacted with the acid chloride of 4-chlorobutyric acid, to give the amide (3). This is then used to alkylate para-fluorophenylpiperazine (4) to yield monatepil.[2][3]
References
edit- ^ Sugimoto T, Hosoki K, Karasawa T (July 1995). "Relative contribution of alpha 1-adrenoceptor blocking activity to the hypotensive effect of the novel calcium antagonist monatepil". Journal of Cardiovascular Pharmacology. 26 (1): 55–60. doi:10.1097/00005344-199507000-00009. PMID 7564365. S2CID 1548014.
- ^ a b US patent 4749703, Hitoshi Uno, et al., "Calcium antagonist piperazine derivatives, and compositions therefor", issued 1988-06-07, assigned to Dainippon Pharmaceutical Co Ltd
- ^ Kurokawa, Mikio; Sato, Fuminori; Fujiwara, Iwao; et al. (1991). "A new class of calcium antagonists. 2. Synthesis and biological activity of 11-[4-[4-(4-fluorophenyl)-1-piperazinyl]butyryl]amino]-6,11-dihydrodibenzo[b,e]thiepin maleate and related compounds". Journal of Medicinal Chemistry. 34 (3): 927–934. doi:10.1021/jm00107a009. PMID 2002473.