Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor[5] which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.[6]
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.211 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1746 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BrF3 | |
| Molar mass | 136.90 g/mol |
| Appearance | straw-coloured liquid hygroscopic |
| Odor | Choking, pungent[1] |
| Density | 2.803 g/cm3 [2] |
| Melting point | 8.77 °C (47.79 °F; 281.92 K) |
| Boiling point | 125.72 °C (258.30 °F; 398.87 K) |
| Reacts with water[3] | |
| Structure | |
| T-shaped (C2v) | |
| 1.19 D | |
| Hazards[4] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Reacts violently with water to release HF, highly toxic, corrosive, powerful oxidizer |
| GHS labelling: | |
   
| |
| Danger | |
| H271, H300+H310+H330, H314, H373 | |
| P102, P103, P210, P220, P221, P260, P264, P271, P280, P283, P284, P301+P310, P301+P330+P331, P303+P361+P353, P304+P312, P305+P351+P338+P310, P306+P360, P308+P313, P340, P363, P370+P380 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | http://www.chammascutters.com/en/downloads/Bromine-Trifluoride-MSDS.pdf |
| Related compounds | |
Other anions
|
Bromine monochloride |
Other cations
|
Chlorine trifluoride Iodine trifluoride |
Related compounds
|
Bromine monofluoride Bromine pentafluoride |
| Supplementary data page | |
| Bromine trifluoride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editBromine trifluoride was first described by Paul Lebeau in 1906, who obtained the material by the reaction of bromine with fluorine at 20 °C:[7]
- Br2 + 3 F2 → 2 BrF3
The disproportionation of bromine monofluoride also gives bromine trifluoride:[5]
- 3 BrF → BrF3 + Br2
Structure
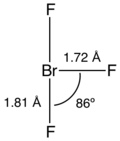
editLike ClF3 and IF3, the BrF3 molecule is T-shaped and planar. In the VSEPR formalism, the bromine center is assigned two electron lone pairs. The distance from the bromine atom to each axial fluorine atom is 1.81 Å and to the equatorial fluorine atom is 1.72 Å. The angle between an axial fluorine atom and the equatorial fluorine atom is slightly smaller than 90° — the 86.2° angle observed is due to the repulsion generated by the electron pairs being greater than that of the Br-F bonds.[8][9]
Chemical properties
editIn a highly exothermic reaction, BrF3 reacts with water to form hydrobromic acid and hydrofluoric acid:
- BrF3 + 2 H2O → 3 HF + HBr + O2
BrF3 is a fluorinating agent, but less reactive than ClF3.[10] Already at -196 °C, it reacts with acetonitrile to give 1,1,1-trifluoroethane.[11]
- BrF3 + CH3CN → CH3CF3 + 1⁄2 Br2 + 1⁄2 N2
The liquid is conducting, owing to autoionisation:[6]
- 2 BrF3 ⇌ BrF+2 + BrF−4
Fluoride salts dissolve readily in BrF3 forming tetrafluorobromate:[6]
- KF + BrF3 → KBrF4
It reacts as a fluoride donor:[12]
- BrF3 + SbF5 → [BrF+2][SbF−6]
References
edit- ^ "Safety Data Sheet : Bromine Trifluoride" (PDF). Chammascutters.com. Retrieved 2022-03-17.
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 2012-05-13. Retrieved 2012-11-25.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ "Safety Data Sheet Bromine Trifluoride" (PDF). Airgas. Retrieved 16 January 2020.
- ^ a b Simons JH (1950). "Bromine(III) Fluoride (Bromine Trifluoride)". Bromine (III) Fluoride - Bromine Trifluoride. Inorganic Syntheses. Vol. 3. pp. 184–186. doi:10.1002/9780470132340.ch48. ISBN 978-0-470-13234-0.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Lebeau P. (1906). "The effect of fluorine on chloride and on bromine". Annales de Chimie et de Physique. 9: 241–263.
- ^ Gutmann V (1950). "Die Chemie in Bromitrifluorid". Angewandte Chemie. 62 (13–14): 312–315. Bibcode:1950AngCh..62..312G. doi:10.1002/ange.19500621305.
- ^ Meinert H (1967). "Interhalogenverbindungen". Zeitschrift für Chemie. 7 (2): 41–57. doi:10.1002/zfch.19670070202.
- ^ Rozen, Shlomo; Sasson, Revital (2007). "Bromine Trifluoride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/9780470842898.rb266.pub2. ISBN 978-0471936237.
- ^ Rozen, Shlomo (2010). "Selective Reactions of Bromine Trifluoride in Organic Chemistry". Advanced Synthesis & Catalysis. 352 (16): 2691–2707. doi:10.1002/adsc.201000482.
- ^ A. J. Edwards and G. R. Jones. J. Chem. Soc. A, 1467 (1969)
