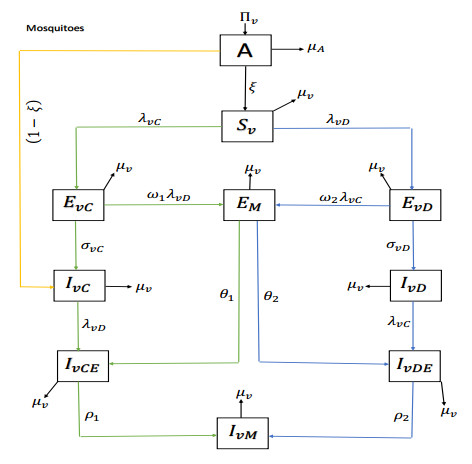
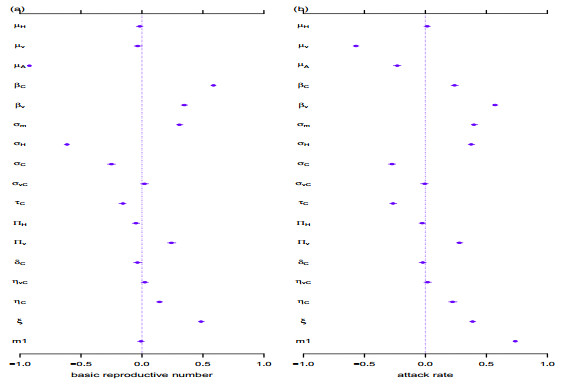
|
[1]
|
F. B. Agusto, S. Easley, K. Freeman and M. Thomas, Mathematical model of three age-structured transmission dynamics of Chikungunya virus, Journal of Computational and Mathematical Methods in Medicine, (2016), Art. ID 4320514, 31 pp.
doi: 10.1155/2016/4320514.


|
|
[2]
|
J. A. Ayukekbong, Dengue virus in nigeria: Current status and future perspective, British Journal of Virology, 1 (2014), 106-111.

|
|
[3]
|
J. Carr, Application of Centre Manifold Theory, Applied Mathematical Sciences, 35. Springer-Verlag, New York-Berlin, 1981.


|
|
[4]
|
C. Castillo-Chavez and B. J. Song, Dynamical model of tuberclosis and their applications, Mathematical Bioscience Engineering, 1 (2004), 361-404.
doi: 10.3934/mbe.2004.1.361.


|
|
[5]
|
D. Cecilia, Current status of dengue and chikungunya in India, WHO South-East Asia Journal of Public Health, 3 (2014), 22-26.
doi: 10.4103/2224-3151.206879.

|
|
[6]
|
N. Chitnis, J. M. Cushing and J. M. Hyman, Bifurcation analysis of a mathematical model for malaria transmission, SIAM Journal on Applied Mathematics, 67 (2006), 24-45.
doi: 10.1137/050638941.


|
|
[7]
|
H. Delatte, G. Gimonneau, A. Triboire and D. Fontenille, Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean, Journal of Medical Entomology, 46 (2009), 33–41, https://doi.org/10.1603/033.046.0105.

|
|
[8]
|
Y. Dumont and F. Chiroleu, Vector control for the Chikungunya disease, Mathematical Bioscience Engineering, 7 (2010), 315-348.
doi: 10.3934/mbe.2010.7.313.


|
|
[9]
|
Y. Dumont, F. Chiroleu and C. Domerg, On a temporal model for the chikungunya disease: Modeling theory and numerics, Mathematical Bioscience, 213 (2008), 80-91.
doi: 10.1016/j.mbs.2008.02.008.


|
|
[10]
|
Y. Dumont and J. M. Tchuenche, Mathematical studies on the strile insect technique for the Chikungunya disease and Aedes albopictus, Journal of Mathematical Biology, 65 (2012), 809-854.
doi: 10.1007/s00285-011-0477-6.


|
|
[11]
|
V. Duong, L. Lambrechts, R. E. Paul, S. Ly, R. S. Lay, K. C. Long, R. Huy, A. Tarantola, T. W. Scott, A. Sakuntabhai and P. Buchy, Asymptomatic humans transmit dengue virus to mosquitoes, PNAS, 112 (2015), 14688-14693.
doi: 10.1073/pnas.1508114112.

|
|
[12]
|
First Dengue Vaccine Approved in More than 10 Countries by Sanofi Pasteur 2019., Available from: https://www.sanofipasteur.com/en/.

|
|
[13]
|
L. Furuya-Kanamori, S. H. Lian, G. Milinovic, R. J. S. Magalhaes, A. C. A. Clements, W. B. Hu, P. Brasil, F. D. Frentiu, R. Dunning and L. Yakob, Co-distribution and co-infection of chikungunya and dengue viruses, BMC Infectious Diseases, 16 (2016), 11 pp.
doi: 10.1186/s12879-016-1417-2.

|
|
[14]
|
B. S. Gandhi, K. Kulkarni, M. Gobele, S. S. Dole, S. Kapur and P. Satpathy, et al., Dengue and chikungunya co-infection associated with more severe clinical disease than mono-infection, International J. of Healthcare and Biomedical Research, 3 (2015), 117-123.

|
|
[15]
|
D. Gao, Y. Lou, D. He, T. C. Porco, Y. Kuang, G. Chowell and S. G. Ruan, Prevention and control of zika as a mosquito-borne and sexually transmitted disease: A mathematical modeling analysis, Scientific Report, 6 (2016), 28070.
doi: 10.1038/srep28070.

|
|
[16]
|
S. M. Garba, A. B. Gumel and M. R. Abu Bukar, Backward bifurcations in dengue transmission dynamics, Mathematical Biosciences, 215 (2008), 11-25.
doi: 10.1016/j.mbs.2008.05.002.


|
|
[17]
|
D. J. Gubler, E. E. Ooi, S. G. Vasudevan and J. Farrar, Dengue and dengue Hemorrhagic Fever, 2nd ed. Wallingford, UK: CAB International, 2014.

|
|
[18]
|
D. J. Gubler, Dengue and dengue hemorrhagic fever, Clinical Microbiology Reviews, 11 (1998), 480–496, https://doi.org/10.1128/CMR.11.3.480.

|
|
[19]
|
A. B. Gumel, Causes of backward bifurcations in some epidemiological models, Journal of Mathematical Analysis and Applications, 395 (2012), 355-365.
doi: 10.1016/j.jmaa.2012.04.077.


|
|
[20]
|
N. Hussaini, J.M.-S Lubuma, K. Barley and A. B. Gumel, Mathematical analysis of a model for AVL-HIV co-endemicity, Mathematical Biosciences, 271 (2016), 80-95.
doi: 10.1016/j.mbs.2015.10.008.


|
|
[21]
|
N. Hussaini, K. Okuneye and A. B. Gumel, Mathematical analysis of a model for zoonotic visceral leishmaniasis, Infectious Disease Modelling, 2 (2017), 455-474.
doi: 10.1016/j.idm.2017.12.002.

|
|
[22]
|
J. Jain, R. B. S. Kushwaha, S. S. Singha, A. Sharma, A. Adakb, O. P. Singh, R. K. Bhatnagar, S. K. Snbbarao and S. Sunil, Evidence for natural vertical transmission of chikungunya viruses in field populations of Aedes aegypti in Delhi and Haryana states in India—A preliminary report, Acta. Tropica., 162 (2016), 46-55.
doi: 10.1016/j.actatropica.2016.06.004.

|
|
[23]
|
R. M. Lana, T. G. S. Carneiro, N. A. Hono'rio and C. T. Code, Seasonal and nonseasonal dynamics of Aedes aegypti in Rio de Janeiro, Brazil: Fitting mathematical models to trap data, Acta Tropica, 129 (2014), 25–32, https://doi.org/10.1016/j.actatropica.2013.07.025.

|
|
[24]
|
J. P. LaSalle, The Stability of Dynamical Systems, Regional Conference Series in Applied Mathematics, Society for Industrial and Applied Mathematics, Philadelphia, Pa., 1976.


|
|
[25]
|
R. R. Mahale, A. Mehta, A. K. Shankar and R. Srinivasa, Delayed subdural hematoma after recovery from dengue shock syndrome, Journal of Neurosciences in Rural Practice, 7 (2016), 323-324.
doi: 10.4103/0976-3147.178655.

|
|
[26]
|
C. A. Manore, K. S. Hickman, S. Xu, H. J. Wearing and J. M. Hyman, Comparing dengue and chikungunya emergence and endemic transmission in A. aegypti and A. albopictus, Journal of Theoretical Biology, 356 (2014), 174-191.
doi: 10.1016/j.jtbi.2014.04.033.


|
|
[27]
|
E. Massad, S. Ma, M. N. Burattini, Y. Tun, F. A. B. Coutinho and L. W. Ang, The risk of chikungunya fever in a dengue-endemic area, Journal of Travel Medicine, 15 (2008), 147-155.
doi: 10.1111/j.1708-8305.2008.00186.x.

|
|
[28]
|
D. Moulay, M. A. Aziz-Alaoui and M. Cadivel, The chikungunya disease: Modeling, vector and transmission global dynamics, Mathematical Biosciences, 229 (2011), 50-63.
doi: 10.1016/j.mbs.2010.10.008.


|
|
[29]
|
S. S. Musa, S. Zhao, H. S. chan, Z. Jin and D. He, A mathematical model to study the 2014-2015 large-scale dengue epidemics in Kaohsiung and Tainan cities in Taiwan, China, Mathematical Biosciences Engineering, 16 (2019), 3841–3863, http://dx.doi.org/10.3934/mbe.2019190.

|
|
[30]
|
S. Naowarat, W. Tawarat and I. M. Tang, Control of the transmission of chikungunya fever epidemic through the use of adulticide, Americal Journal of Applied Sciences, 8 (2011), 558–565, http://repository.li.mahidol.ac.th/dspace/handle/123456789/12916.
doi: 10.3844/ajassp.2011.558.565.

|
|
[31]
|
National Vector Borne Disease Control Programme, 22, Shamnath Marg, Delhi 110054, 2018, http://nvbdcp.gov.in/index4.php?lang=1&level=0&linkid=486&lid=3765 and http://nvbdcp.gov.in/index4.php?lang=1&level=0&linkid=431&lid=3715.

|
|
[32]
|
S. Nimmannitya, S. B. Halstead, S. N. Cohen and M. R. Margiotta, Dengue and chikungunya virus infection in Man in Thailand 1962–1964. I. Observations on hospitalized patients with hemorrhagic fever, The American Journal of Tropical Medicine and Hygiene, 18 (1969), 954–971, https://doi.org/10.4269/ajtmh.1969.18.954.

|
|
[33]
|
N. Nuraini, E. Soewono and K. A. Sidarto, Mathematical model of dengue disease transmission with severe DHF compartment, BULLETIN of the Malaysian Mathematical Sciences Society, 30 (2007), 143–157, http://math.usm.my/bulletin.


|
|
[34]
|
K. Okuneye and A. B. Gumel, Analysis of a temperature- and rainfall-dependent model for malaria transmission dynamics, Mathematical Biosciences, 287 (2017), 72-92.
doi: 10.1016/j.mbs.2016.03.013.


|
|
[35]
|
K. O. Okuneye, J. X. Valesco-Hernandez and A. B. Gumel, The "unholy" chikungunya-dengue-zika trinity: A theoretical analysis, Journal of Biological Systems, 25 (2017), 545-585.
doi: 10.1142/S0218339017400046.


|
|
[36]
|
K. Pesko, C. Westbrook, C. Mores, L. Lounibos and M. Reiskind, Effects of infectious virus dose and blood meal delivery method on susceptibility of Aedes aegypti and Aedes albopictus to chikungunya virus, Journal of Medical Entomology, 46 (2009), 395–399, https://doi.org/10.1603/033.046.0228.

|
|
[37]
|
E. Pliego Pliego, J. Velazquez-Castro and A. Fraguela Collar, Seasonality on the life cycle of Aedes aegypti mosquito and its statistical relation with dengue outbreaks, Applied Mathematical Modelling, 50 (2017), 484-496.
doi: 10.1016/j.apm.2017.06.003.


|
|
[38]
|
S. Sang, S. Gu, P. Bi, W. Yang, Z. Yang, L. Xu and et al., Predicting unprecedented dengue outbreak using imported cases and climatic factors in Guangzhou, PLoS Neglected Tropical Diseases, 9 (2014), e0003808, https://doi.org/10.1371/journal.pntd.0003808.

|
|
[39]
|
T. Saswat, A. Kumar, S. Kumar, P. Mamidi, S. Muduli and N. K. Debata, et al., High rates of co-infection of dengue and Chikungunya virus in Odisha and Maharashtra, India during 2013, Infectious, Genetics and Evolution, 35 (2015), 134-141.
doi: 10.1016/j.meegid.2015.08.006.

|
|
[40]
|
The World Bank Data, Life Expectancy at Birth website 2019., Available from: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=IN.

|
|
[41]
|
The World Bank Data, Population website 2019. Available from: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=NG-IN&name_desc=true.

|
|
[42]
|
M. Turell, J. Beaman and R. Tammariello, Susceptibility of selected strains of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to chikungunya virus, Journal of Medical Entomology, 29 (1992), 49–53, https://doi.org/10.1093/jmedent/29.1.49.

|
|
[43]
|
S. Usaini, U. T. Mustapha and S. S. Musa, Modelling scholastic underachievement as a contagious disease, Mathematical Methods in the Applied Sciences, 41 (2018), 8603-8612.
doi: 10.1002/mma.4924.


|
|
[44]
|
P. van den Driessche and J. Watmough, Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission, Mathematical Biosciences, 180 (2002), 29-48.
doi: 10.1016/S0025-5564(02)00108-6.


|
|
[45]
|
K. S. Vannice, A. Durbin and J. Hombach, Status of vaccine research and development of vaccines for dengue, Vaccine, 34 (2016), 2934-2938.
doi: 10.1016/j.vaccine.2015.12.073.

|
|
[46]
|
S. F. Wang, W. H. Wang, K. Chang, Y. H. Chen, S. P. Tseng, C. H. Yen, D.C. Wu and Y. M. Chen, Severe dengue fever outbreak in Taiwan, American Journal of Tropical Medicine and Hygiene, 94 (2016), 193-197.
doi: 10.4269/ajtmh.15-0422.

|
|
[47]
|
World Heath Organization (WHO), the Chikungunya factsheet 2016. Available from: http://www.who.int/mediacentre/factsheets/fs327/en/.

|
|
[48]
|
World Heath Organization, Dengue fact sheet 2017. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/.

|
|
[49]
|
L. Yakob and A. C. A. Clements, A Mathematical model of chikungunya dynamics and control: The major epidemic on re'union Island, PLoS ONE, 8 (2013), e57448.
doi: 10.1371/journal.pone.0057448.

|
|
[50]
|
H. M. Yang, M. L. G. Macoris, K. C. Galvani, M. T. M. Andrighetti and D. M. V. Wanderley, Assesseing the effectcts of temperature on the population of Aedes aegypti, the vector of dengue, Epidemiology and Infection, 137 (2009), 1188–1202, https://doi.org/10.1017/S0950268809002040.

|
|
[51]
|
L. Zou, J. Chen, X. M. Feng and S. G. Ruan, Analysis of a dengue model with vertical transmission and application to the 2014 dengue outbreak in Guangdong Province, China. Bulletin of Mathematical Biology, 80 (2018), 2633-2651.
doi: 10.1007/s11538-018-0480-9.


|








 Download:
Download: