Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author(s): Hyun Kyoung Lee, Medical Registrar; and Louise Reiche, Dermatologist, Palmerston North, NZ (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction Demographics Causes Clinical features Complications Diagnosis Treatment Prevention Outcome
Panton-Valentine leukocidin (PVL) is an exotoxin produced by certain strains of Staphylococcus aureus (SA).
Staph. aureus with a virulence factor, such as the PVL exotoxin, enhances infection transmission and treatment resistance. PVL-SA is highly transmissible, lyses human neutrophils, and may cause recurrent skin and soft tissue infections (SSTIs) despite antibiotic treatment.
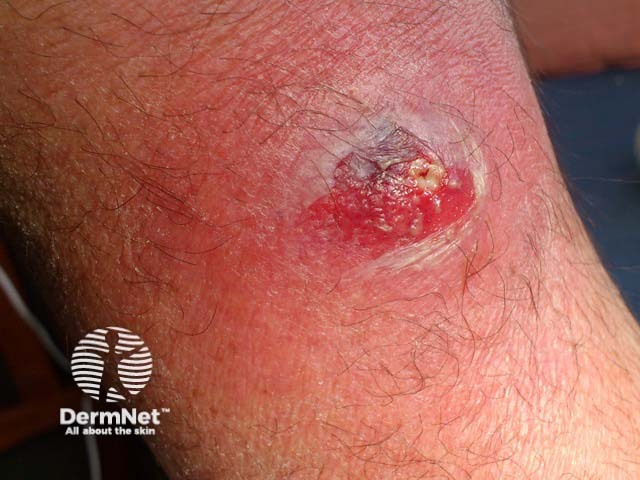
A large painful boil which on culture showed PVL-SA
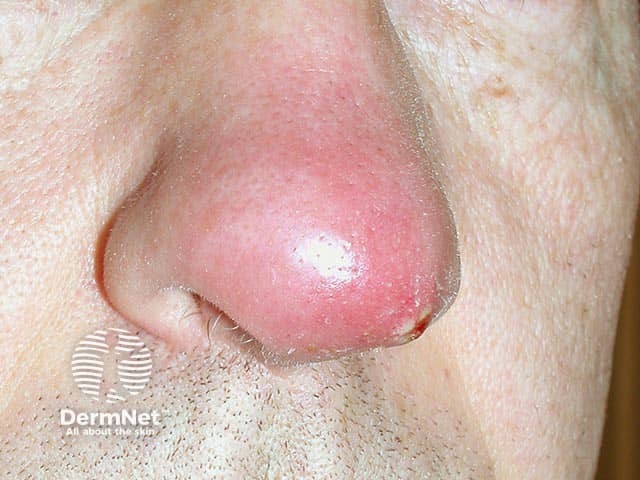
A nasal furuncle due to a PVL Staphylococcus
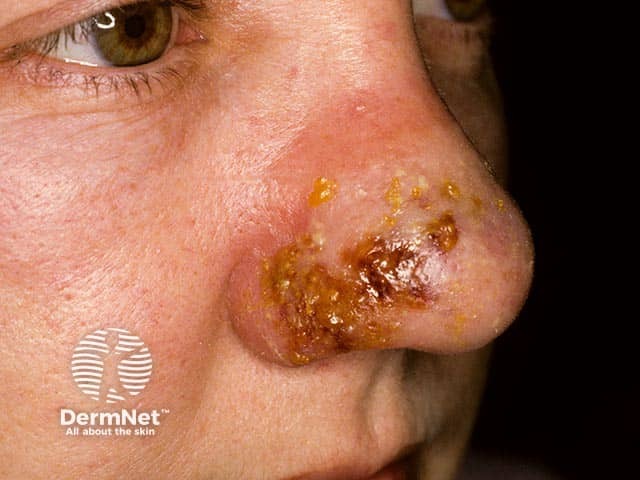
Nasal cellulitis due to a PVL staphylococcal infection
The disease prevalence of PVL-SA varies worldwide, for example 19% in Europe, 29% in the USA, 28–54% in Australia, and 47–75% in Western Africa. These figures may be an underestimate as PVL-SA is not routinely tested in laboratories unless specifically requested.
Global dissemination has been enhanced through international travel. Patients with PVL-SA are often young adults with minimal exposure to healthcare settings.
The main risk factors for acquiring PVL SA, known as the ‘five Cs’ are:
The Panton-Valentine leukocidin (PVL) toxin induces cytolytic pores in human polymorphonuclear neutrophils (PMNs), monocytes, and macrophages which in turn induces apoptosis. Two proteins comprise the PVL cytotoxin: LukS-PV and LukF-PV, encoded by their namesake genes. LukS-PV and LukF-PV are carried on bacteriophages.
Cell death is toxin concentration dependent. Neutrophil apoptosis in the peripheral circulation weakens bacterial defence. This may explain the increased severity of necrotising pneumonia for PVL-positive patients, who have a higher frequency of neutropaenia.
Another contributing factor to the PVL toxin virulence is the upregulation of pro-inflammatory cytokines and nuclear factor-kappa B (NF-κB) in neutrophils, independently increasing necrotising infections.
PVL-SA clinical presentations vary widely ranging from asymptomatic nasopharyngeal carriage, skin and soft tissue infections (SSTIs), to necrotising pneumonia.
Distinctive features for PVL-positive (compared to PVL-negative) Staph. aureus include:
The most common PVL-SA SSTIs are:
PVL-SA can cause invasive infections in immunocompetent individuals, such as:
PVL-SA should be clinically suspected if there are recurrent abscesses/boils or necrotising SSTIs in an otherwise healthy individual, especially if a close contact is similarly affected.
Send bacterial swabs from the nose and infected areas of skin, and samples (eg, sputum, pus, or exudate) from other affected areas (as there may be coinfection) for microscopy, culture, and antibiotic sensitivities, specifically requesting PVL testing.
The PVL virulence factor can be found in both methicillin-sensitive Staph. aureus, ie, MSSA strains (9–46%) and methicillin-resistant Staph. aureus ie, MRSA (74–100%).
For cases of necrotising pneumonia, test for influenza due to the risk of coinfection.
Consider prompt anti-staphylococcal regimen and an antitoxin agent; change to an appropriate antibiotic once susceptibilities are known.
Important hygiene measures for infected patients, close contacts, and carers include:
Specific treatment for PVL-SA depends on clinical disease severity at presentation.
PVL-SA preventative measures include hygiene and decolonisation measures for patients, close contacts, and those sharing the same household.
PVL-SA patients and close contacts who are treated early typically recover well. Clinician awareness, specific laboratory testing, and early appropriate decolonisation and antimicrobial use are key.
PVL-positive musculoskeletal infections generally have greater severity of inflammation and are more likely to require surgical intervention.
For necrotising pneumonia, the mortality rate in PVL-positive patients can be up to 75%, compared to 36% in PVL-negative cases.