Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Amanda Oakley, Department of Dermatology, Waikato Hospital, Hamilton, New Zealand, 1997. Updated February 2016. Updated May 2021
" data-index="1" alt="Go to Introduction
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(1 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Introduction
" data-index="2" alt="Go to Uses
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(2 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Uses
" data-index="3" alt="Go to Risk in pregnancy and during breastfeeding
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(3 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Risk in pregnancy and during breastfeeding
" data-index="4" alt="Go to Mechanism
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(4 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Mechanism
" data-index="5" alt="Go to Side effects and risks
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(5 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Side effects and risks
Interactions
Acitretin is an oral retinoid (vitamin-A derivative) used to treat severe psoriasis, usually at a dose of 0.25–1 mg per kg body weight per day. It is best taken after a meal because it needs fat to be absorbed through the gut wall.
Acitretin is available as 10 mg and 25 mg capsules. Trade names include Neotigason™ and Novatretin®. Since March 2009, PHARMAC funding in New Zealand requires Special Authority application by a dermatologist or vocationally registered general practitioner. Restrictions apply.
Acitretin is particularly useful for some types of psoriasis including pustular psoriasis, erythrodermic psoriasis, and psoriasis affecting hands and feet. It is not effective for psoriatic arthritis.
It is occasionally used to treat other skin conditions including:
Acitretin MUST NOT be taken in pregnancy; it can damage an unborn child and cause congenital disabilities. Strict birth control measures must be used during treatment and for three years after stopping acitretin. Therefore, acitretin is rarely prescribed to females of child-bearing potential. If it is, they will be asked to have a blood pregnancy test before treatment and regularly during treatment. People on acitretin should not donate blood during treatment or for three years afterwards. Acitretin is also contraindicated while breastfeeding.
It does not affect male sexual function or offspring, so males of all ages can take it.
Acitretin is a metabolite of an earlier antipsoriatic retinoid, etretinate. Etretinate (Tigason™) is no longer available in New Zealand.
Acitretin is thought to work in psoriasis by slowing down the proliferation of the skin cells. A response is noted in more than half of treated patients. Improvement begins about two weeks after starting treatment and is maximum after about twelve weeks. The affected skin either peels off or gradually clears.
Some patients are treated with acitretin for a few months, repeated from time to time, while others remain on the acitretin long term.
In resistant cases, acitretin can be combined with other antipsoriatic drugs and phototherapy.
Acitretin has side effects that may limit the dose that can be used.
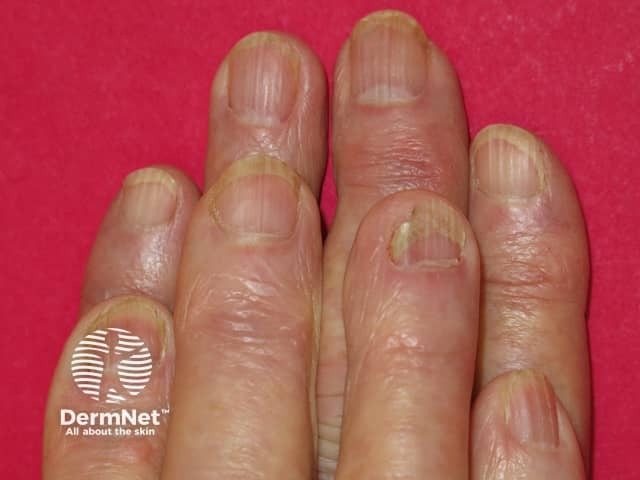
Acitretin nail thinning
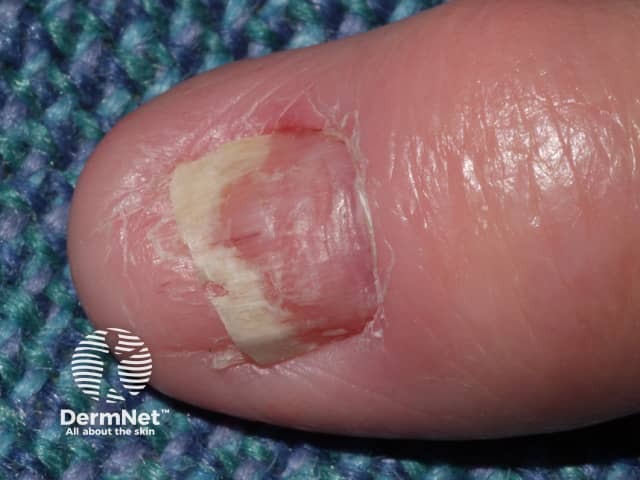
Acitretin nail thinning
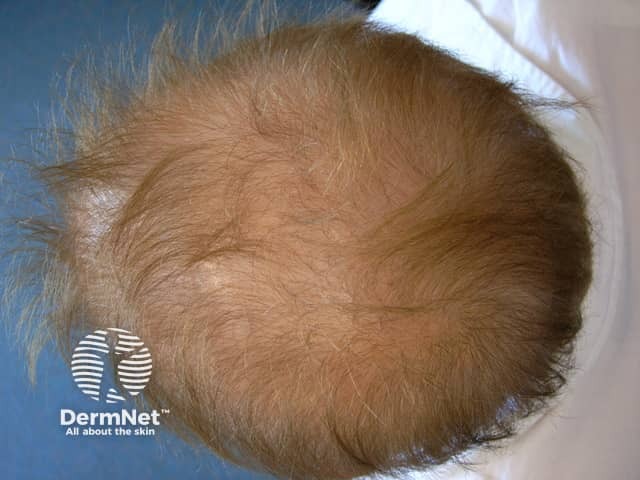
Acitretin diffuse hair loss
Acitretin should not usually be taken at the same time as the following medications (there may be rare exceptions):
It is best to avoid alcohol when on acitretin, especially if triglyceride levels are high.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).