Description for Eligard
ELIGARD® is a sterile polymeric matrix formulation of leuprolide acetate, a GnRH agonist, for subcutaneous injection. It is designed to deliver leuprolide acetate at a controlled rate over a one-, three-, four- or six-month therapeutic period.
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone (GnRH) that, when given continuously, inhibits pituitary gonadotropin secretion and suppresses testicular and ovarian steroidogenesis. The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-Dleucyl- L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
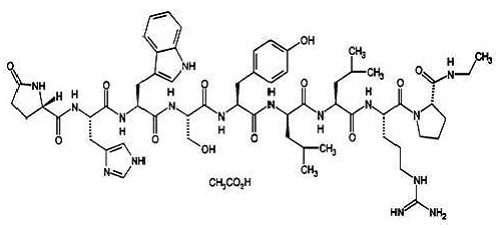 |
ELIGARD® is prefilled and supplied in two separate, sterile syringes whose contents are mixed immediately prior to administration. The two syringes are joined and the single dose product is mixed until it is homogenous. ELIGARD® is administered subcutaneously, where it forms a solid drug delivery depot.
One syringe contains the ATRIGEL Delivery System and the other contains leuprolide acetate. ATRIGEL is a polymeric (non-gelatin containing) delivery system consisting of a biodegradable poly (DL-lactide-co-glycolide) (PLGH or PLG) polymer formulation dissolved in a biocompatible solvent, N-methyl-2-pyrrolidone (NMP).
Refer to Table 5 for the delivery system composition and constituted product formulation for each ELIGARD® product.
Table 5: ELIGARD® Delivery System Composition and Constituted Product Formulation
| ELIGARD® | 7.5 mg | 22.5 mg | 30 mg | 45 mg | |
| ATRIGEL® Delivery System syringe | Polymer | PLGH | PLG | PLG | PLG |
| Polymer description | Copolymer containing carboxyl endgroups | Copolymer with hexanediol | Copolymer with hexanediol | Copolymer with hexanediol | |
| Polymer DL-lactide to glycolide molar ratio | 50:50 | 75:25 | 75:25 | 85:15 | |
| Constituted product | Polymer delivered | 82.5 mg | 158.6 mg | 211.5 mg | 165 mg |
| NMP delivered | 160.0 mg | 193.9 mg | 258.5 mg | 165 mg | |
| Leuprolide acetate delivered | 7.5 mg | 22.5 mg | 30 mg | 45 mg | |
| Approximate Leuprolide free base equivalent | 7.0 mg | 21 mg | 28 mg | 42 mg | |
| Approximate administered formulation weight | 250 mg | 375 mg | 500 mg | 375 mg | |
| Approximate injection volume | 0.25 mL | 0.375 mL | 0.5 mL | 0.375 mL | |
Uses for Eligard
ELIGARD is indicated for the treatment of advanced prostate cancer.
Dosage for Eligard
Recommended Dosage
ELIGARD is administered subcutaneously and provides continuous release of leuprolide acetate over a one-, three-, four-, or six-month treatment period (Table 1). ELIGARD must be administered by a healthcare provider. The injection delivers the dose of leuprolide acetate incorporated in a polymer formulation.
Table 1: ELIGARD Recommended Dosing
| Dosage | 7.5 mg | 22.5 mg | 30 mg | 45 mg |
| Recommended dose | 1 injection every month | 1 injection every 3 months | 1 injection every 4 months | 1 injection every 6 months |
As with other drugs administered by subcutaneous injection, the injection site should vary periodically. The specific injection location should be an area with sufficient soft or loose subcutaneous tissue. In clinical trials, the injections were administered in the upper-or mid-abdominal area. Avoid areas with brawny or fibrous subcutaneous tissue or locations that could be rubbed or compressed (i.e., with a belt or clothing waistband).
Preparation Instructions
Use aseptic technique throughout the procedure. As with other similar agents, the use of gloves is recommended during mixing and administration.1 Allow the product to reach room temperature before mixing. Once mixed, the product must be administered within 30 minutes or it should be discarded.
ELIGARD is packaged in a carton containing:
- Tray containing pre-connected syringe system and desiccant pack
- Prescribing information
- Sterile safety needle and cap (located under the tray in carton)
Follow the detailed instructions below to ensure correct preparation of ELIGARD prior to administration:
Step 1
On a clean field open the tray by tearing off the foil from the corner and remove the contents. Discard the desiccant pack. Remove the pre-connected syringe system from the tray. Open the sterile safety needle package by peeling back the paper tab. Note: Syringe A and Syringe B should not be lined-up yet. The product should only be administered with the co-packaged, sterile safety needle.
 |
Step 2
Grasp the latching button on the coupling device with your finger and thumb and press until you hear a snapping sound. The two syringes will be aligned.
Do not bend the pre-connected syringe system.
 |
Step 3
Holding the syringes in a horizontal position, transfer the liquid contents of Syringe A into the leuprolide acetate powder contained in Syringe B. Thoroughly mix the product for 60 cycles by pushing the contents back and forth between both syringes to obtain a uniform suspension.
- A cycle is one push of the Syringe A plunger and one push of the Syringe B plunger.
- When thoroughly mixed, the suspension will appear light tan to tan (ELIGARD 7.5 mg) or colorless to pale yellow (ELIGARD 22.5 mg, 30 mg, and 45 mg).
Note: Product must be mixed as described; shaking will NOT provide adequate mixing. Do not bend.
 |
Step 4
After mixing, hold the syringes vertically (upright) with Syringe B (wide syringe) on the bottom. The syringes should remain securely coupled. Transfer all of the mixed product into Syringe B by depressing the Syringe A plunger and slightly withdrawing the Syringe B plunger.
 |
Step 5
While ensuring the Syringe A plunger is fully pushed down, hold the coupling device and unscrew Syringe B. This will disconnect Syringe B from the coupling device. Syringe A will remain attached to the coupling device.
Note: Small air bubbles will remain in the formulation – this is acceptable.
Do not purge the air bubbles from Syringe B as product may be lost!
 |
Step 6
Continue to hold Syringe B upright with the open end at the top. Hold back the white plunger on Syringe B to prevent loss of the product and attach the safety needle and cap. Gently screw clockwise with approximately a three-quarter turn until the safety needle and cap are secure.
Do not overtighten, as the needle hub may become damaged which could result in leakage of the product during injection. The safety shield may also be damaged if the safety needle and cap are screwed with too much force.
 |
Step 7
Move the safety shield away from the needle and towards the syringe.
Pull off the cap immediately prior to administration.
 |
Note: Should the needle hub appear to be damaged, or leak, the product should NOT be used. The damaged safety needle and cap should NOT be replaced and the product should NOT be injected. In the event of damage to the needle hub, use a new replacement ELIGARD carton.
Administration Instructions
 |
 |
- Select an injection site on the abdomen, upper buttocks, or another location with adequate amounts of subcutaneous tissue that does not have excessive pigment, nodules, lesions, or hair and hasn’t recently been used.
- Cleanse the injection-site area with an alcohol swab (not enclosed).
- Using the thumb and forefinger, grab and bunch the area of skin around the injection site.
- Using your dominant hand, insert the needle quickly at a 90° angle to the skin surface. The depth of penetration will depend on the amount and fullness of the subcutaneous tissue and the length of the needle. After the needle is inserted, release the skin.
- Inject the drug using a slow, steady push and press down on the plunger until the syringe is empty. Make sure all the drug has been injected before removing the needle.
- Withdraw the needle quickly at the same 90° angle used for insertion.
- Immediately following the withdrawal of the needle, activate the safety shield using a finger/thumb or flat surface and push until it completely covers the needle tip and locks into place.
- An audible and tactile “click” verifies a locked position.
- Check to confirm the safety shield is fully engaged. Discard all components safely in an appropriate biohazard container.
 |
HOW SUPPLIED
Dosage Forms And Strengths
ELIGARD is an injectable suspension of leuprolide acetate available in a pre-connected syringe system and packaged with a sterile safety needle and cap (Table 2), a desiccant, prescribing information and instructions for use. The pre-connected syringe system consists of syringe A and syringe B connected using a coupling device. Syringe A contains the in situ polymeric extended release technology and the syringe B contains leuprolide acetate powder. When reconstituted, ELIGARD is administered as a single dose. Refer to Table 10 for details of the description of the dosage form before and after mixing [see HOW SUPPLIED/Storage And Handling (16)].
Table 2: Specifications for ELIGARD Sterile Safety Needle and Cap
| ELIGARD strength | Gauge | Length |
| 7.5 mg | 20-gauge | 5/8-inch |
| 22.5 mg | 20-gauge | 5/8-inch |
| 30 mg | 20-gauge | 5/8-inch |
| 45 mg | 18-gauge | 5/8-inch |
Storage And Handling
ELIGARD is supplied as a single-dose, two syringe-mixing system with a sterile safety needle and cap. ELIGARD is available as follows (Table 10):
Table 10: ELIGARD Product Presentations
| ELIGARD strength | NDC number | Description of Syringe A with the delivery system | Description of Syringe B with leuprolide acetate | Description of the suspension after mixing |
| 7.5 mg | 62935-756-80 | Light tan to tan, clear, viscous solution | White to off white powder | Light tan to tan |
| 22.5 mg | 62935-227-10 | Colorless to pale yellow, clear viscous solution | White to off white powder | Colorless to pale yellow |
| 30 mg | 62935-306-40 | Colorless to pale yellow, clear viscous solution | White to off white powder | Colorless to pale yellow |
| 45 mg | 62935-461-50 | Colorless to pale yellow, clear viscous solution | White to off white powder | Colorless to pale yellow |
Storage
Store at 2°C to 8°C (36°F to 46°F)
Once outside the refrigerator this product may be stored in its original packaging at room temperature 15&fdeg;C to 30°C (59°F to 86°F) for up to eight weeks prior to mixing and administration.
Manufactured by: Tolmar, Fort Collins, CO 80526. Revised: Jan 2024
Side Effects for Eligard
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Tumor Flare [see WARNINGS AND PRECAUTIONS]
- Hyperglycemia and Diabetes [see WARNINGS AND PRECAUTIONS]
- Cardiovascular Disease [see WARNINGS AND PRECAUTIONS]
- Effect on QT/QTc Interval [see WARNINGS AND PRECAUTIONS]
- Convulsions [see WARNINGS AND PRECAUTIONS]
Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of all ELIGARD formulations was evaluated in clinical trials involving patients with advanced prostate cancer. In addition, the safety of ELIGARD 7.5 mg was evaluated in 8 surgically castrated males (Table 4). ELIGARD, like other GnRH analogs, caused a transient increase in serum testosterone concentrations during the first one to two weeks of treatment. Therefore, potential exacerbations of signs and symptoms of the disease during the first weeks of treatment are of concern in patients with vertebral metastases and/or urinary obstruction or hematuria. If these conditions are aggravated, it may lead to neurological problems such as weakness and/or paresthesia of the lower limbs or worsening of urinary symptoms [see WARNINGS AND PRECAUTIONS].
During the clinical trials, injection sites were closely monitored. Refer to Table 3 for a summary of reported injection site adverse reactions.
Table 3: Reported Injection Site Adverse Reactions
| ELIGARD | 7.5 mg | 22.5 mg | 30 mg | 45 mg |
| Study number | AGL9904 | AGL9909 | AGL0001 | AGL0205 |
| Number of patients | 120 | 117 | 90 | 111 |
| Treatment | 1 injection every month up to 6 months | 1 injection every 3 months up to 6 months | 1 injection every 4 months up to 8 months | 1 injection every 6 months up to 12 months |
| Number of injections | 716 | 230 | 175 | 217 |
| Transient burning/stinging | 248 (34.6%) injections; 84% reported as mild | 50 (21.7%) injections; 86% reported as mild | 35 (20%) injections; 100% reported as mild3 | 35 (16%) injections; 91.4% reported as mild |
| Pain (generally brief and mild) | 4.3% of injections (18.3% of patients) | 3.5% of injections (6.0% of patients) | 2.3% of injections2 (3.3% of patients) | 4.6% of injections4 |
| Erythema (generally brief and mild) | 2.6% of injections (12.5% of patients) | 0.9% of injections1 (1.7% of patients) | 1.1% of injections (2.2% of patients) | - |
| Bruising (mild) | 2.5% of injections (11.7% of patients) | 1.7% of injections (3.4% of patients) | - | 2.3% of injections5 |
| Pruritus | 1.4% of injections (9.2% of patients) | 0.4% of injections (0.9% of patients) | - | - |
| Induration | 0.4% of injections (2.5% of patients) | - | - | - |
| Ulceration | 0.1% of injections (> 0.8% of patients) | - | - | - |
| 1Erythema was reported following 2 injections of ELIGARD 22.5 mg. One report characterized the erythema as mild and it resolved within 7 days. The other report characterized the erythema as moderate and it resolved within 15 days. Neither patient experienced erythema at multiple injection times. 2A single reaction reported as moderate pain resolved within two minutes and all 3 mild pain reactions resolved within several days following injection of ELIGARD 30 mg. 3Following injection of ELIGARD 30 mg, three of the 35 burning/stinging reactions were reported as moderate. 4Transient pain was reported as mild in intensity in nine of ten (90%) events and moderate in intensity in one of ten (10%) events following injection of ELIGARD 45 mg. 4Mild bruising was reported following 5 (2.3%) study injections and moderate bruising was reported following 2 (<1%) study injections of ELIGARD 45 mg. |
||||
These localized adverse reactions were non-recurrent over time. No patient discontinued therapy due to an injection site adverse reaction.
The following possibly or probably related systemic adverse reactions occurred during clinical trials with ELIGARD, and were reported in > 2% of patients (Table 4). Reactions considered not drug-related are excluded.
Table 4: Summary of Possible or Probably Related Systemic Adverse Reactions Reported by > 2% of Patients Treated with ELIGARD
| ELIGARD | 7.5 mg | 7.5 mg | 22.5 mg | 30 mg | 45 mg | |
| Study number | AGL9904 | AGL9802 | AGL9909 | AGL0001 | AGL0205 | |
| Number of patients | 120 | 8 | 117 | 90 | 111 | |
| Treatment | 1 injection every month up to 6 months | 1 injection (surgically castrated patients) | 1 injection every 3 months up to 6 months | 1 injection every 4 months up to 8 months | 1 injection every 6 months up to 12 months | |
| Body system | Adverse Reaction | Number (percent) | ||||
| Body as a whole | Malaise and fatigue | 21 (17.5%) | - | 7 (6.0%) | 12 (13.3%) | 13 (11.7%) |
| Weakness | - | - | - | - | 4 (3.6%) | |
| Nervous system | Dizziness | 4 (3.3%) | - | - | 4 (4.4%) | - |
| Vascular | Hot flashes/sweats | 68 (56.7%)* | 2 (25.0%)* | 66 (56.4%)* | 66 (73.3%)* | 64 (57.7%)* |
| Renal/urinary | Urinary frequency | - | - | 3 (2.6%) | 2 (2.2%) | - |
| Nocturia | - | - | - | 2 (2.2%) | - | |
| Gastrointestinal | Nausea | - | - | 4 (3.4%) | 2 (2.2%) | - |
| Gastroenteritis/ colitis | 3 (2.5%) | - | - | - | - | |
| Skin | Pruritus | - | - | 3 (2.6%) | - | - |
| Clamminess | - | - | - | 4 (4.4%)* | - | |
| Night sweats | - | - | - | 3 (3.3%)* | 3 (2.7%)* | |
| Alopecia | - | - | - | 2 (2.2%) | - | |
| Musculoskeletal | Arthralgia | - | - | 4 (3.4%) | - | - |
| Myalgia | - | - | - | 2 (2.2%) | 5 (4.5%) | |
| Pain in limb | - | - | - | - | 3 (2.7%) | |
| Reproductive | Testicular atrophy | 6 (5.0%)* | - | - | 4 (4.4%)* | 8 (7.2%)* |
| Gynecomastia | - | - | - | 2 (2.2%)* | 4 (3.6%)* | |
| Testicular pain | - | - | - | 2 (2.2%) | - | |
| Psychiatric | Decreased libido | - | - | - | 3 (3.3%)* | - |
| *Expected pharmacological consequences of testosterone suppression. In the patient populations studied with ELIGARD 7.5 mg, a total of 86 hot flashes/sweats adverse reactions were reported in 70 patients. Of these, 71 reactions (83%) were mild; 14 (16%) were moderate; 1 (1%) was severe. In the patient population studied with ELIGARD 22.5 mg, a total of 84 hot flashes/sweats adverse reactions were reported in 66 patients. Of these, 73 reactions (87%) were mild; 11 (13%) were moderate; none were severe. In the patient population studied with ELIGARD 30 mg, a total of 75 hot flash adverse reactions were reported in 66 patients. Of these, 57 reactions (76%) were mild; 16 (21%) were moderate; 2 (3%) were severe. In the patient population studied with ELIGARD 45 mg, a total of 89 hot flash adverse reactions were reported in 64 patients. Of these, 62 reactions (70%) were mild; 27 (30%) were moderate; none were severe. |
||||||
In addition, the following possibly or probably related systemic adverse reactions were reported by < 2% of the patients treated with ELIGARD in these clinical studies.
| Body system | Adverse Reactions |
| General | Sweating, insomnia, syncope, rigors, weakness, lethargy |
| Gastrointestinal | Flatulence, constipation, dyspepsia |
| Hematologic | Decreased red blood cell count, hematocrit and hemoglobin |
| Metabolic | Weight gain |
| Musculoskeletal | Tremor, backache, joint pain, muscle atrophy, limb pain |
| Nervous | Disturbance of smell and taste, depression, vertigo |
| Psychiatric | Insomnia, depression, loss of libido* |
| Renal/urinary | Difficulties with urination, pain on urination, scanty urination, bladder spasm, blood in urine, urinary retention, urinary urgency, incontinence, nocturia, nocturia aggravated |
| Reproductive/ Urogenital | Testicular soreness/pain, impotence*, decreased libido*, gynecomastia*, breast soreness/tenderness*, testicular atrophy*, erectile dysfunction, penile disorder*, reduced penis size |
| Skin | Alopecia, clamminess, night sweats*, sweating increased* |
| Vascular | Hypertension, hypotension |
| * Expected pharmacological consequences of testosterone suppression. | |
Changes In Bone Density
Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with a GnRH agonist analog. It can be anticipated that long periods of medical castration in men will have effects on bone density.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ELIGARD. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a casual relationship to drug exposure.
Pituitary apoplexy-During postmarketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
Nervous System-Convulsions
Respiratory System-Interstitial lung disease
Drug Interactions for Eligard
No Information provided
Warnings for Eligard
Included as part of the PRECAUTIONS section.
Precautions for Eligard
Tumor Flare
ELIGARD 7.5 mg, 22.5 mg, and 30 mg like other GnRH agonists, causes a transient increase in serum concentrations of testosterone during the first week of treatment. ELIGARD 45 mg causes a transient increase in serum concentrations of testosterone during the first two weeks of treatment. Patients may experience worsening of symptoms or onset of new signs and symptoms during the first few weeks of treatment, including bone pain, neuropathy, hematuria, or bladder outlet obstruction.
Cases of ureteral obstruction and/or spinal cord compression, which may contribute to paralysis with or without fatal complications, have been observed in the treatment of advanced prostate cancer using GnRH agonists.
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy. If spinal cord compression or ureteral obstruction develops, standard treatment of these complications should be instituted.
Laboratory Tests
Monitor ELIGARD response by periodic measurement of serum concentrations of testosterone and prostate specific antigen.
In the majority of patients, testosterone levels increased above Baseline during the first week, declining thereafter to Baseline levels or below by the end of the second or third week. Castrate levels were generally reached within two to four weeks.
Castrate testosterone levels were maintained for the duration of the treatment with ELIGARD 7.5 mg. No increases to above the castrate level occurred in any of the patients.
Castrate levels were generally maintained for the duration of treatment with ELIGARD 22.5 mg.
Once castrate levels were achieved with ELIGARD 30 mg, most (86/89) patients remained suppressed throughout the study.
Once castrate levels were achieved with ELIGARD 45 mg, one patient (< 1%) experienced a breakthrough, with testosterone levels > 50 ng/dL.
Results of testosterone determinations are dependent on assay methodology. It is advisable to be aware of the type and precision of the assay methodology to make appropriate clinical and therapeutic decisions.
Drug/Laboratory Test Interactions
Therapy with ELIGARD results in suppression of the pituitary-gonadal system. Results of diagnostic tests of pituitary gonadotropic and gonadal functions conducted during and after ELIGARD therapy may be affected.
Hyperglycemia And Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
Effect On QT/QTc Interval
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
Convulsions
Postmarketing reports of convulsions have been observed in patients on leuprolide acetate therapy. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above. Patients receiving a GnRH agonist who experience convulsion should be managed according to current clinical practice.
Embryo-Fetal Toxicity
Based on findings in animal studies and mechanism of action, ELIGARD may cause fetal harm when administered to a pregnant woman. In animal developmental and reproductive studies, major fetal abnormalities were observed after administration of leuprolide acetate throughout gestation in rats. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus [see Use In Specific Populations, CLINICAL PHARMACOLOGY].
Patient Counseling Information
Hypersensitivity
- Inform patients that if they have experienced hypersensitivity with other GnRH agonist drugs like ELIGARD, ELIGARD is contraindicated [see CONTRAINDICATIONS].
Tumor Flare
- Inform patients that ELIGARD can cause tumor flare during the first weeks of treatment. Inform patients that the increase in testosterone can cause an increase in urinary symptoms or pain. Advise patients to contact their healthcare provider if ureteral obstruction, spinal cord compression, paralysis, or new or worsened symptoms occur after beginning ELIGARD treatment [see WARNINGS AND PRECAUTIONS].
Hyperglycemia And Diabetes
- Advise patients that there is an increased risk of hyperglycemia and diabetes with ELIGARD therapy. Inform patients that periodic monitoring for hyperglycemia and diabetes is required when being treated with ELIGARD [see WARNINGS AND PRECAUTIONS].
Cardiovascular Disease
- Inform patients that there is an increased risk of myocardial infarction, sudden cardiac death, and stroke with ELIGARD treatment. Advise patients to immediately report signs and symptoms associated with these events to their healthcare provider for evaluation [see WARNINGS AND PRECAUTIONS].
Injection Site Reactions
- Inform patients that injection site related adverse reactions may occur such as transient burning/stinging, pain, bruising, and redness. Advise patients to contact their healthcare provider if they experience rash or severe injection site reactions [see ADVERSE REACTIONS].
Urogenital Disorders
- Advise patients that ELIGARD may cause impotence.
Infertility
- Inform patients that ELIGARD may cause infertility [see Use In Specific Populations].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Two-year carcinogenicity studies were conducted with leuprolide acetate in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate-induced tumors or pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years. Patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities. No carcinogenicity studies have been conducted with ELIGARD.
Mutagenicity studies have been performed with leuprolide acetate using bacterial and mammalian systems and with ELIGARD 7.5 mg in bacterial systems. These studies provided no evidence of a mutagenic potential.
Use In Specific Populations
Pregnancy
Risk Summary
Based on findings in animal studies and mechanism of action, ELIGARD may cause fetal harm when administered to a pregnant woman [see CLINICAL PHARMACOLOGY]. There are no available data in pregnant women to inform the drug-associated risk. Expected hormonal changes that occur with ELIGARD treatment increase the risk for pregnancy loss. In animal developmental and reproductive studies, major fetal abnormalities were observed after administration of leuprolide acetate throughout gestation in rats. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus (see Data).
Data
Animal Data
In animal developmental and reproductive studies, major fetal abnormalities were observed after administration of leuprolide acetate throughout gestation. There were increased fetal mortality and decreased fetal weights in rats and rabbits. The effects of fetal mortality are expected consequences of the alterations in hormonal levels brought about by this drug.
Lactation
The safety and efficacy of ELIGARD have not been established in females. There is no information regarding the presence of ELIGARD in human milk, the effects on the breastfed child, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in a breastfed child from ELIGARD, a decision should be made to discontinue breastfeeding or discontinue the drug, taking into account the importance of the drug to the mother.
Females And Males Of Reproductive Potential
Infertility
Males
Based on mechanism of action, ELIGARD may impair fertility in males of reproductive potential [see CLINICAL PHARMACOLOGY].
Pediatric Use
The safety and effectiveness of ELIGARD in pediatric patients have not been established.
Geriatric Use
The majority of the patients (approximately 70%) studied in the clinical trials were age 70 and older. Clinical studies of ELIGARD did not include sufficient numbers of younger adult patients to determine if patients 65 years of age and older respond differently than younger adult patients.
Overdose Information for Eligard
No Information provided
Contraindications for Eligard
Hypersensitivity
ELIGARD is contraindicated in patients with hypersensitivity to GnRH, GnRH agonist analogs or any of the components of ELIGARD. Anaphylactic reactions to synthetic GnRH or GnRH agonist analogs have been reported in the literature.
Clinical Pharmacology for Eligard
Mechanism Of Action
Leuprolide acetate, a gonadotropin releasing hormone (GnRH) agonist, acts as an inhibitor of gonadotropin secretion when given continuously in therapeutic doses. Animal and human studies indicate that after an initial stimulation, chronic administration of leuprolide acetate results in suppression of testicular and ovarian steroidogenesis. This effect is reversible upon discontinuation of drug therapy.
Pharmacodynamics
Following initial administration, ELIGARD causes an initial increase in serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels with subsequent increases in serum levels of testosterone. Administration of ELIGARD leads to sustained suppression of pituitary gonadotropins, and serum levels of testosterone consequently fall into the range normally seen in surgically castrated men approximately 21 days after initiation of therapy. Continuation of therapy with leuprolide acetate in patients with prostate cancer maintained testosterone below the castrate level for up to five years.
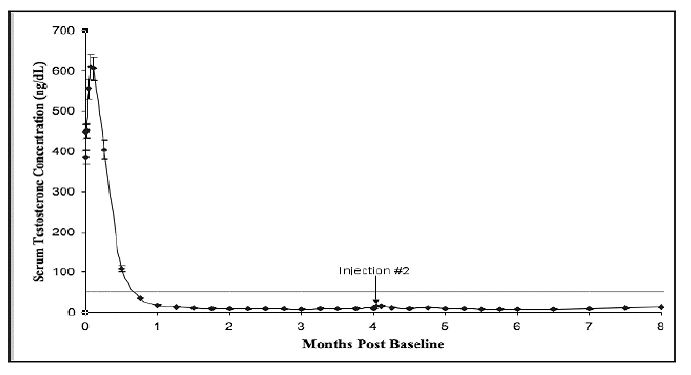
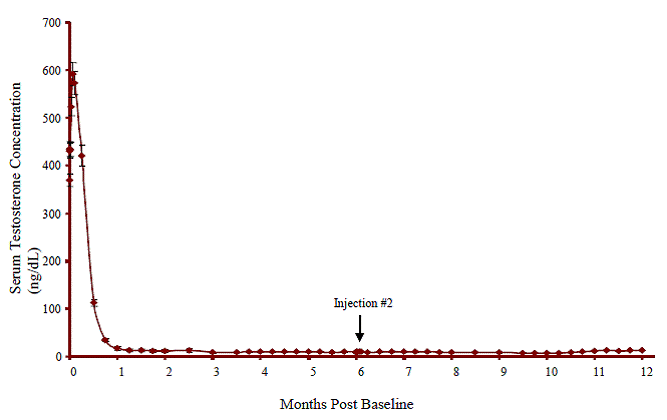
Following the first dose of ELIGARD®, mean serum testosterone concentrations transiently increased, then fell to below castrate threshold (≤ 50 ng/dL) within three weeks, and generally remained below castrate threshold levels throughout treatment for all ELIGARD® concentrations. Mean serum testosterone concentrations fell to ≤ 20 ng/dL within five weeks for all ELIGARD® concentrations.
Pharmacokinetics
Absorption
ELIGARD® 7.5 mg
Following a single injection of ELIGARD® 7.5 mg for 1-month administration in patients, the mean peak plasma concentration was 25.3 ng/mL (Cmax) at 5 hours;at the end of the 4 week dosing interval the mean concentration declined to 0.42 ng/mL.
ELIGARD® 22.5 mg
Following a single injection of ELIGARD® 22.5 mg for 3-month administration in patients, the mean peak plasma concentration was 127 ng/mL (Cmax) at 5 hours; at the end of the 12 week dosing interval the mean concentration declined to 0.34 ng/mL .
ELIGARD® 30 mg
Following a single injection of ELIGARD® 30 mg for 4-month administration in patients, the mean peak plasma concentration was 150 ng/mL (Cmax) at 3.3 hours; at the end of the 16 week dosing interval the mean concentration declined to 0.1 ng/mL.
ELIGARD® 45 mg
Following a single injection of ELIGARD® 45 mg for 6-month administration in patients, the mean peak plasma concentration was 82 ng/mL at 4.5 hours (Cmax); at the end of the 24 week dosing interval the mean concentration declined to 0.2 ng/mL.
There was no evidence of significant accumulation during repeated dosing.
Distribution
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male volunteers was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Elimination
After the initial increase following each subcutaneous injection, serum concentration range was 0.1 – 2.00 ng/mL.
In healthy male volunteers, a 1-mg bolus of leuprolide administered intravenously revealed that the mean systemic clearance was 8.34 L/h, with a terminal elimination half-life of approximately 3 hours based on a two compartment model.
Metabolism
No drug metabolism study was conducted with ELIGARD®. Upon administration with different leuprolide acetate formulations, the major metabolite of leuprolide acetate is a pentapeptide (M- 1) metabolite.
Excretion
No drug excretion study was conducted with ELIGARD®.
Specific Populations
Geriatrics [see Use In Specific Populations]
Race
In patients studied, mean serum leuprolide concentrations were similar regardless of race. Refer to Table 7 for distribution of study patients by race.
Table 7. Race Characterization of ELIGARD® Study Patients
| Race | ELIGARD® 7.5 mg |
ELIGARD® 22.5 mg |
ELIGARD® 30 mg |
ELIGARD® 45 mg |
| White | 26 | 19 | 18 | 17 |
| Black | - | 4 | 4 | 7 |
| Hispanic | 2 | 2 | 2 | 3 |
Renal And Hepatic Insufficiency
The pharmacokinetics of ELIGARD® in hepatically and renally impaired patients have not been determined.
Clinical Studies
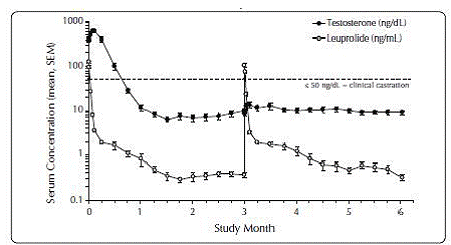
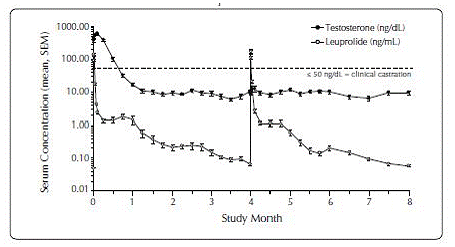
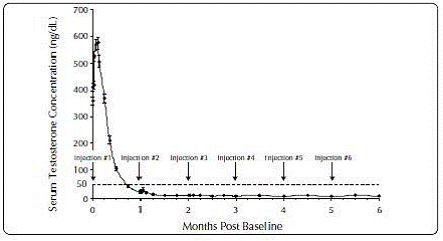
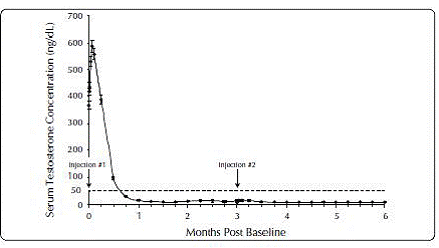
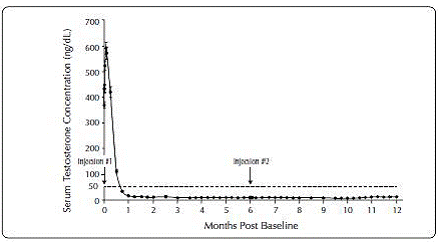
One open-label, multicenter study was conducted with each ELIGARD® formulation (7.5 mg, 22.5 mg, 30 mg, and 45 mg) in patients with Jewett stage A though D prostate cancer who were treated with at least a single injection of study drug (Table 8). The efficacy outcome measure was medical castration rate, defined as achieving and maintaining serum testosterone suppression to ≤ 50 ng/dL (Figures 1-4).
During the AGL9904 study using ELIGARD® 7.5 mg, once testosterone suppression below 50 ng/dL was achieved, no patients (0%) demonstrated breakthrough (concentration > 50 ng/dL) at any time in the study.
During the AGL9909 study using ELIGARD® 22.5 mg, once testosterone suppression below 50 ng/dL was achieved, one patient (< 1%) demonstrated breakthrough following the initial injection; that patient remained below 50 ng/dL following the second injection.
During the AGL0001 study using ELIGARD® 30 mg, once testosterone suppression below 50 ng/dL was achieved, three patients (3%) demonstrated breakthrough. In the first of these patients, a single serum testosterone concentration of 53 ng/dL was reported on the day after the second injection. In this patient, suppression below 50 ng/dL was reported for all other time points. In the second patient, a serum testosterone concentration of 66 ng/dL was reported immediately prior to the second injection. This rose to a maximum concentration of 147 ng/dL on the second day after the second injection. In this patient, suppression below 50 ng/dL was again reached on the seventh day after the second injection and was maintained thereafter. In the final patient, serum testosterone concentrations > 50 ng/dL were reported at 2 and at 8 hours after the second injection. Serum testosterone concentration rose to a maximum of 110 ng/dL on the third day after the second injection. In this patient, suppression below 50 ng/dL was again reached eighteen days after the second injection and was maintained until the final day of the study, when a single serum testosterone concentration of 55 ng/dL was reported.
During the AGL0205 study using ELIGARD® 45 mg, once testosterone suppression below 50 ng/dL was achieved, one patient (<1%) demonstrated breakthrough. This patient reached castrate suppression below 50 ng/dL at Day 21 and remained suppressed until Day 308 when his testosterone level rose to 112 ng/dL. At Month 12 (Day 336), his testosterone was 210 ng/dL.
Table 8. Summary of ELIGARD® Clinical Studies
| ELIGARD® | 7.5 mg | 22.5 mg | 30 mg | 45 mg | |
| Study number | AGL9904 | AGL9909 | AGL0001 | AGL0205 | |
| Total number of patients | 120 (117 completed) | 1172 (111 completed3) | 90 (82 completed4) | 111 (103 completed5) | |
| Jewett stages | Stage A | - | 2 | 2 | 5 |
| Stage B | - | 19 | 38 | 43 | |
| Stage C | 89 | 60 | 16 | 19 | |
| Stage D | 31 | 36 | 34 | 44 | |
| Treatment | 6 monthly injections | 1 injection (4 patients) | 1 injection (5 patients) | 1 injection (5 patients) | |
| 2 injections, one every three months (113 patients) | 2 injections, oneevery four months (85 patients) | 2 injections,one everysix months (106 patients) | |||
| Duration of therapy | 6 months | 6 months | 8 months | 12 months | |
| Mean testosterone concentration (ng/dL) | Baseline | 361.3 | 367.1 | 385.5 | 367.7 |
| Day 2 | 574.6 (Day 3) | 588.0 | 610.0 | 588.6 | |
| Day 14 | Below Baseline(Day 10) | Below Baseline | Below Baseline | Below Baseline | |
| Day 28 | 21.8 | 27.7 (Day 21) | 17.2 | 16.7 | |
| Conclusion | 6.1 | 10.1 | 12.4 | 12.6 | |
| Number of patients with testosterone ≤ 50ng/dL | Day 28 | 112 of 119(94.1%) | 115 of 116(99%) | 85 of 89 (96%) | 108 of 109 (99.1%) |
| Day 35 | - | 116 (100%) | - | - | |
| Day 42 | 118 (100%) | - | 89 (100%) | - | |
| Conclusion | 1171 (100%) | 111 (100%) | 81 (99%) | 102 (99%) | |
| Number of patients with testosterone≤ 20 ng/dL | Day 28 | 90 of 119 (76%) | 94 of 116 (81%) | 60 of 89 (67%) | 87 of 109 (80%) |
| 1. Two patients withdrew for reasons unrelated to drug. 2. One patient received less than a full dose at Baseline, never attained castration, and was withdrawn at Day 73 and given an alternate treatment. 3. All non-evaluable patients who attained castration by Day 28 maintained castration at each time point up toand including the time of withdrawal. 4. One patient withdrew on Day 14. All 7 non-evaluable patients who had achieved castration by Day 28 maintained castration at each time point, up to and including the time of withdrawal. 5. Two patients were withdrawn prior to the Month 1 blood draw. One patient did not achieve castration and was withdrawn on Day 85. All 5 non-evaluable patients who attained castration by Day 28, maintainedcastration at each time point up to and including the time of withdrawal. |
|||||
Figure 1. ELIGARD® 7.5 mg Mean Serum Testosterone Concentrations (n=117)
 |
Figure 2. ELIGARD® 22.5 mg Mean Serum Testosterone Concentrations (n=111)
 |
Figure 3. ELIGARD® 30 mg Mean Serum Testosterone Concentrations (n=90)
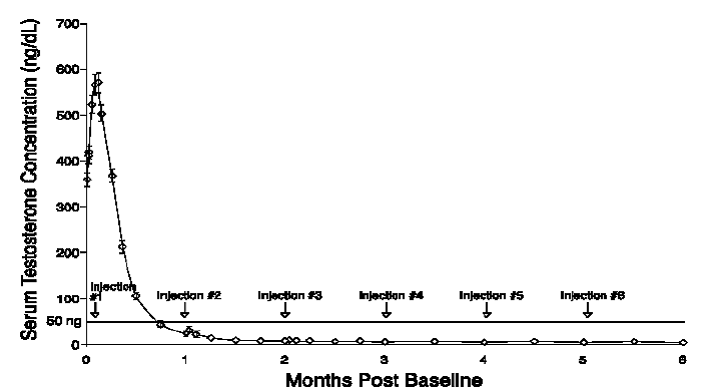 |
Figure 4. ELIGARD® 45 mg Mean Serum Testosterone Concentrations (n=103)
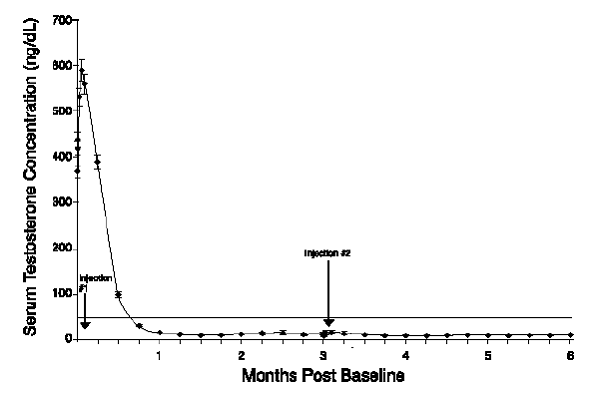 |
Serum PSA decreased in all patients in all studies whose Baseline values were elevated above the normal limit. Refer to Table 9 for a summary of the effectiveness of ELIGARD® in reducing serum PSA values.
Table 9. Effect of ELIGARD® on Patient Serum PSA Values
| ELIGARD® | 7.5 mg | 22.5 mg | 30 mg | 45 mg |
| Mean PSA reduction at study conclusion | 94% | 98% | 86% | 97% |
| Patients with normal PSA at study conclusion* | 94% | 91% | 93% | 95% |
| *Among patients who presented with elevated levels at Baseline | ||||
Other secondary efficacy endpoints evaluated included WHO performance status, bone pain, urinary pain and urinary signs and symptoms. Refer to Table 10 for a summary of these endpoints.
Table 10. Secondary Efficacy Endpoints
| ELIGARD® | 7.5 mg | 22.5 mg | 30 mg | 45 mg | |
| Baseline | WHO Status = 01 | 88% | 94% | 90% | 90% |
| WHO Status = 12 | 11% | 6% | 10% | 7% | |
| WHO Status = 23 | - | - | - | 3% | |
| Mean bone pain4 (range) | 1.22 (1-9) | 1.20 (1-9) | 1.20 (1-7) | 1.38 (1-7) | |
| Mean urinary pain (range) | 1.12 (1-5) | 1.02 (1-2) | 1.01 (1-2) | 1.22 (1-8) | |
| Mean urinary signsand symptoms (range) | Low | 1.09 (1-4) | Low | Low | |
| Number of patients with prostate abnormalities | 102 (85%) | 96 (82%) | 66 (73%) | 89 (80%) | |
| Month 6 | Month 6 | Month 8 | Month 12 | ||
| Follow-up | WHO status = 0 | Unchanged | 96% | 87% | 94% |
| WHO status = 1 | Unchanged | 4% | 12% | 5% | |
| WHO status = 2 | - | - | 1% | 1% | |
| Mean bone pain (range) | 1.26 (1-7) | 1.22 (1-5) | 1.19 (1-8) | 1.31 (1-8) | |
| Mean urinary pain (range) | 1.07 (1-8) | 1.10 (1-8) | 1.00 (1-1) | 1.07 (1-5) | |
| Mean urinary signsand symptoms (range) | Modestly decreased | 1.18 (1-7) | Modestly decreased | Modestly decreased | |
| Number of patients with prostate abnormalities | 77 (64%) | 76 (65%) | 54 (60%) | 60 (58%) | |
| 1. WHO status = 0 classified as “fully active.” 2. WHO status = 1 classified as “restricted in strenuous activity but ambulatory and able to carry out work of alight or sedentary nature.” 3. WHO status = 2 classified as “ambulatory but unable to carry out work activities.” 4. Pain score scale: 1 (no pain) to 10 (worst pain possible). |
|||||
Patient Information for Eligard
INSTRUCTIONS FOR USE
Follow the detailed instructions below to ensure correct preparation of ELIGARD prior to administration:
Step 1
Use aseptic technique throughout the procedure. Gloves are recommended during mixing and administration.
Allow the product to reach room temperature before mixing. Once mixed, the product must be administered within 30 minutes or it should be discarded.
On a clean field open the tray by tearing off the foil from the corner and remove the contents. Discard the desiccant pack. Remove the pre-connected syringe system from the tray. Open the sterile safety needle package by peeling back the paper tab. Note: Syringe A and Syringe B should not be lined-up yet. The product should only be administered with the co-packaged, sterile safety needle.
 |
Step 2
Grasp the latching button on the coupling device with your finger and thumb and press until you hear a snapping sound. The two syringes will be aligned.
Do not bend the pre-connected syringe system.
 |
Step 3
Holding the syringes in a horizontal position, transfer the liquid contents of Syringe A into the leuprolide acetate powder contained in Syringe B. Thoroughly mix the product for 60 cycles by pushing the contents back and forth between both syringes to obtain a uniform suspension.
- A cycle is one push of the Syringe A plunger and one push of the Syringe B plunger.
- When thoroughly mixed, the suspension will appear light tan to tan (ELIGARD 7.5 mg) or colorless to pale yellow (ELIGARD 22.5 mg, 30 mg, and 45 mg).
Note: Product must be mixed as described; shaking will NOT provide adequate mixing. Do not bend.
 |
Step 4
After mixing, hold the syringes vertically (upright) with Syringe B (wide syringe) on the bottom. The syringes should remain securely coupled. Transfer all of the mixed product into Syringe B by depressing the Syringe A plunger and slightly withdrawing the Syringe B plunger.
 |
Step 5
While ensuring the Syringe A plunger is fully pushed down, hold the coupling device and unscrew Syringe B. This will disconnect Syringe B from the coupling device. Syringe A will remain attached to the coupling device.
Note: Small air bubbles will remain in the formulation – this is acceptable.
Do not purge the air bubbles from Syringe B as product may be lost!
 |
Step 6
Continue to hold Syringe B upright with the open end at the top. Hold back the white plunger on Syringe B to prevent loss of the product and attach the safety needle and cap. Gently screw clockwise with approximately a three-quarter turn until the safety needle and cap are secure.
Do not overtighten, as the needle hub may become damaged which could result in leakage of the product during injection. The safety shield may also be damaged if the safety needle and cap are screwed with too much force.
 |
Step 7
Move the safety shield away from the needle and towards the syringe.
Pull off the cap immediately prior to administration.
 |
Note: Should the needle hub appear to be damaged, or leak, the product should NOT be used. The damaged safety needle and cap should NOT be replaced and the product should NOT be injected. In the event of damage to the needle hub, use a new replacement ELIGARD carton.
Follow the detailed instructions below to ensure correct administration of ELIGARD:
1. Select an injection site on the abdomen, upper buttocks, or another location with adequate amounts of subcutaneous tissue that does not have excessive pigment, nodules, lesions, or hair and hasn’t recently been used.
2. Cleanse the injection-site area with an alcohol swab (not enclosed).
3. Using the thumb and forefinger, grab and bunch the area of skin around the injection site.
 |
4. Using your dominant hand, insert the needle quickly at a 90° angle to the skin surface. The depth of penetration will depend on the amount and fullness of the subcutaneous tissue and the length of the needle. After the needle is inserted, release the skin.
5. Inject the drug using a slow, steady push and press down on the plunger until the syringe is empty. Make sure all the drug has been injected before removing the needle.
 |
6. Withdraw the needle quickly at the same 90° angle used for insertion.
7. Immediately following the withdrawal of the needle, activate the safety shield using a finger/thumb or flat surface and push until it completely covers the needle tip and locks into place.
8. An audible and tactile “click” verifies a locked position.
9. Check to confirm the safety shield is fully engaged. Discard all components safely in an appropriate biohazard container.
 |
From 

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.