Abstract
Background and aims
Low vitamin D (VITD) may contribute to statin-associated muscle symptoms (SAMS). We examined the influence of baseline and change in VITD in patients with verified SAMS.
Methods
SAMS was verified in 120 patients with prior statin muscle complaints using 8-week randomized, double-blind crossover trials of simvastatin (SIMVA) 20 mg/d and placebo. 25 (OH)vitamin D was measured at each phase of the trial.
Results
Forty-three patients (35.8%) experienced muscle pain on SIMVA but not placebo, exhibiting confirmed SAMS. VITD (mean ± standard deviation) prior to SIMVA treatment were not different between patients who did (31.7 ± 12.1 ng/mL, n=43) or did not (31.6 ± 10.3 ng/mL, n=77) develop SAMS and did not predict SAMS (p=0.96). The change in VITD with SIMVA treatment was not different between patients with and without SAMS (0.3 ± 5.9 vs. 0.2±8.3 ng/mL, respectively) and did not predict SAMS (p=0.96). The proportion of patients classified as VITD deficient (<20 ng/mL) did not differ between patients with (n=16) and without (n=10) SAMS (χ2=1.45; p=0.23), nor did the proportion of patients classified as VITD insufficient (<30 ng/mL) (n=42 vs. 48; χ2<0.01 and p=0.94). Both baseline and on-statin VITD were inversely related to the change in creatine kinase (CK) with statin therapy (p=0.01 and 0.02, respectively), independent of SAMS (p=0.36 and 0.35).
Conclusions
Baseline VITD, VITD deficiency/insufficiency and changes in VITD with statin therapy do not predict SAMS in patients with rigorously verified SAMS. However, low VITD may exacerbate statin-induced muscle injury and could contribute to SAMS development with a longer duration of statin treatment.
Keywords: Statin myalgia, muscle symptoms, Vitamin D deficiency
INTRODUCTION
Statins markedly lower LDL-C and reduce cardiac events. Statins have few side effects but can produce statin-associated muscle symptoms (SAMS) consisting of pain, cramps and weakness in an estimated 5–10% of patients (1–3). SAMS reduce medication compliance and quality of life (4, 5), and 60% of adults who discontinue statins report SAMS as the reason (6).
Several studies and meta-analyses have found a higher prevalence of low vitamin D levels in patients with SAMS (7, 8) and that low Vitamin D is a risk factor for SAMS (9). In addition, vitamin D supplementation may mitigate SAMS (10), but other studies have found no relationship between low vitamin D levels and SAMS (11, 12). Our and others’ results suggest that up to 50% of self-reported SAMS may be non-specific and not attributable to statins (13, 14), which may explain the discrepant results between studies examining the relationship of vitamin D and SAMS.
The present investigation investigated associations between baseline vitamin D levels, and changes in vitamin D with statin therapy, using data from a randomized clinical trial (NCT01140308) in which SAMS were verified using a double-blind, placebo-controlled cross-over design.
MATERIALS AND METHODS
The Coenzyme Q10 in Statin Myopathy trial (NCT01140308) (13, 15) enrolled men and women ≥20 yrs of age with a history of SAMS during statin treatment. Subjects were excluded if they had had cancer within 5 years of entry, hypo- or hyperthyroidism, alanine aminotransferase > 2 UNL), creatinine > 2 mg/dL or were using medications known to affect skeletal muscle metabolism. Subjects using supplemental CoQ10 discontinued supplementation for 2 months before the study.
Subjects were first taken off of cholesterol-lowering drugs for 4 weeks, then entered into a randomized, double-blind, crossover, lead-in trial of SIMVA (20 mg/d) or placebo for 8 weeks to confirm SAMS. Subjects were treated with either the SIMVA or placebo for 8 weeks or until muscle symptoms developed. Study pharmacists compounded identical SIMVA and placebo capsules. Subjects then underwent a 4-week wash-out period, and were crossed over from SIMVA to placebo or vice versa. Muscle symptoms were documented weekly by telephone and were defined as muscle pain that was new/unexplained, unassociated with recent exercise, and persisted for a week (unless intolerable). Only subjects who experienced muscle pain on SIMVA but not on placebo were classified as having confirmed SAMS and entered the subsequent CoQ10 treatment arm of the study. Informed consent was obtained from each patient according to the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Hartford Hospital Institutional Review Board.
Serologic markers
Blood was drawn at each testing time point during the run-in for measurement of serum lipids (total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL)-cholesterol and triglycerides), creatine kinase, 25 (OH)vitamin D, and alanine aminotransferase levels. In addition, creatinine and thyroid stimulating hormone (TSH) levels were measured at baseline in the run-in trial.
Measurement of muscle pain
Subjects in the statin and placebo groups were contacted weekly throughout each phase of the run-in trial to inquire about muscle symptoms using the Brief Pain Inventory (Short Form) (BPI-SF). (16) A pain severity score (PSS) was calculated by averaging scores on 4 pain-intensity items.
Statistical analyses
With a sample size of 120 subjects, a power of 0.80, and an average standard deviation of 10.5 ng/mL in vitamin D, the minimum detectable difference in vitamin D between statin and placebo therapy and/or patients with/without SAMS was 2.7 ng/mL, less than half that observed in previously published literature (8). Standard diagnostics were performed to ensure that basic statistical assumptions, including variance homogeneity, normality, and the presence of outliers, were met prior to data analyses. Vitamin D was either considered as a continuous variable alone and with the inclusion of other potential covariates such as BMI and baseline CK for analysis of variance (ANOVA) models or a categorical variable for chi-square analyses comparing vitamin D levels in patients who did vs. did not develop SAMS. In addition, multilinear regression was used to investigate relationships between continuous variables such as vitamin D, CK, and pain scores in patients with and without SAMS. Seasonal variations for vitamin D were controlled for categorically in models (1 = December–February; 2 = March–May; 3 = June–August; 4 = September–November). All analyses were conducted with the IBM SPSS 22 software package and significance was set at p < 0.05.
RESULTS
The Co Q10 study results have been published (13). In summary, 131 patients were randomized to the cross-over trial; 11 dropped out and 120 completed both phases of the trial. Only 43 (36%) were classified as having confirmed SAMS meaning that they developed muscle pain only on the statin. Of the 77 patients in whom SAMS was not confirmed, 21 (17.5%) reported no pain on either treatment, 21 (17.5%) reported pain on both treatments, and 35 (29%) reported pain on placebo only. Subjects who tested positive for SAMS had a higher BMI and greater CK at study entry (Table 1).
Table 1. Subject characteristics.
Baseline characteristics of subjects who were or were not confirmed for SAMS.
| Confirmed (n=43) | Not confirmed (n=77) | |
|---|---|---|
| Women (n) | 16 | 35 |
| Age (yr) | 58 ± 11 | 61 ± 9 |
| BMI (kg/m2) | 29.6 ± 5.3a | 27.8 ± 4.2 |
| SBP (mmHg) | 122 ± 14 | 124 ± 14 |
| DBP (mmHg) | 75 ± 7 | 75 ± 6 |
| CK (U/L) | 152 ± 89a | 117 ± 68 |
| Total C (mg/dL) | 255 ± 53 | 255 ± 49 |
| LDL-C (mg/dL) | 166 ± 49 | 165 ± 43 |
| HDL-C (mg/dL) | 52 ± 16 | 55 ± 15 |
| Triglycerides (mg/dL) | 186 ± 128 | 173 ± 111 |
Significant difference between groups at p < 0.05.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CK, creatine kinase; C, colesterol; LDL, low density lipoprotein; HDL, high density lipoprotein.
Vitamin D levels before and after statin therapy and placebo were not different in patients with and without confirmed SAMS (all p > 0.45) (Table 2). There was a significant impact of season on post-SIMVA vitamin D levels and changes in vitamin D with statin therapy that was not observed with placebo (Fig. 1) or in either group before treatment (p = 0.59 and 0.64, respectively). However, there were no interactions between SAMS status and season on vitamin D levels with placebo or statin therapy (all p > 0.19).
Table 2. Study vitamin D levels.
Vitamin D levels (ng/mL) before and after statin and placebo therapy in patients with and without confirmed SAMS.
| Confirmed (n=43) | Not confirmed (n=77) | |
|---|---|---|
| Pre-placebo | 30.6 ± 10.3 | 31.9 ± 11.5 |
| Post-placebo | 31.5 ± 11.3 | 31.6 ± 11.6 |
| Δ placebo | 0.5 ± 4.7 | −0.3 ± 5.3 |
| Pre-statin | 31.7 ± 12.1 | 31.5 ± 10.3 |
| Post-statin | 31.4 ± 10.8 | 31.8 ± 10.7 |
| Δ statin | 0.3 ± 5.9 | 0.2 ± 8.3 |
Δ indicates difference between post- to pre-levels. There were no significant differences between groups.
Fig. 1. Seasonal variations in vitamin D.
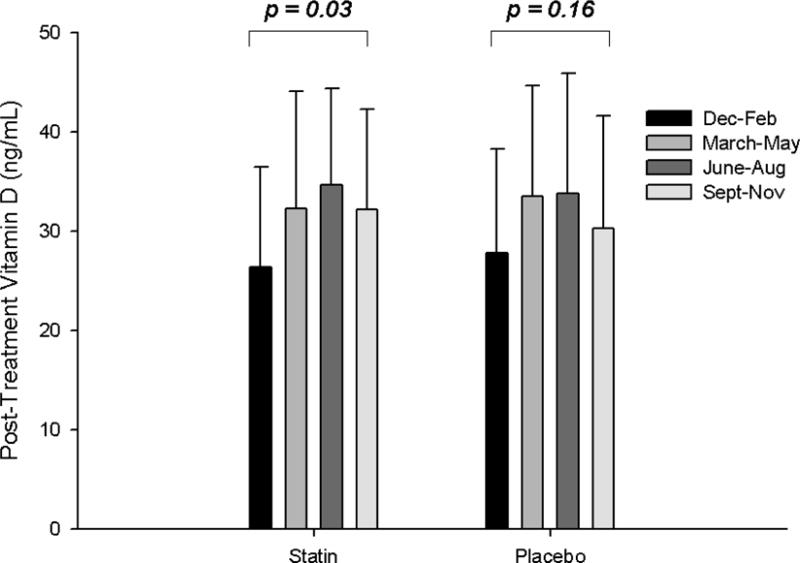
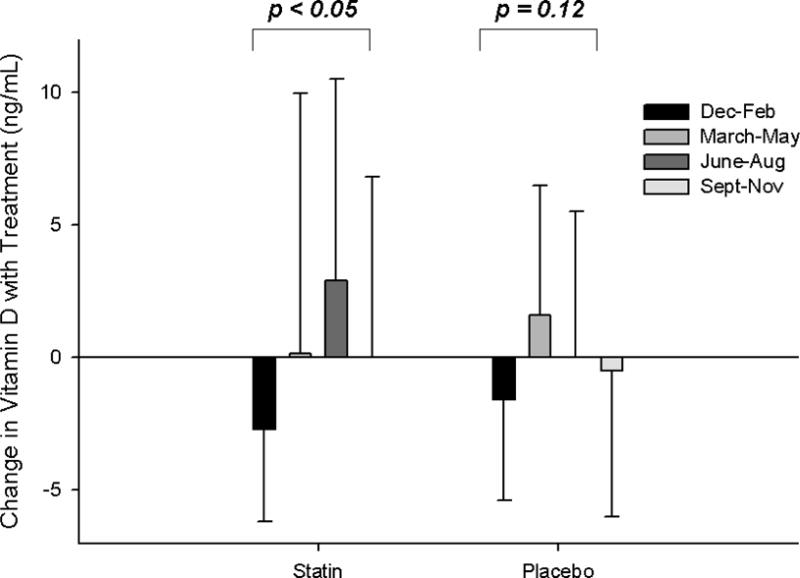
Group means (± standard deviation) of 25 (OH)vitamin D levels post-statin and placebo therapy (A) and as changes with statin and placebo therapy (B), categorized by season.
When patients were classified as vitamin D deficient (vitamin D levels pre-statin therapy < 20 ng/mL) or vitamin D insufficient (vitamin D levels pre-statin therapy < 30 ng/mL), there was no impact of either deficiency (χ2=1.45; p = 0.23) or insufficiency (χ2 < 0.01; p = 0.94) on the development of SAMS (Fig. 2).
Fig. 2. Impact of clinical vitamin D classifications on SAMS.

Percent of patients with vitamin D deficiency (< 20 ng/mL; black shading) and vitamin D insufficiency (< 30 ng/mL; black shading) who did (n=43) vs. did not develop (n=77) confirmed SAMS.
Univariate correlations between pre- and post-levels and changes (Δ) in vitamin D with Δ in pain severity score (PSS) and Δ in CK indicated a significant, inverse relationship between both baseline (Pearson coefficient = −0.24; p = 0.01) and post-treatment (Pearson coefficient = −0.22; p = 0.02) vitamin D levels and Δ CK with statin therapy (Fig. 3). These relationships were not observed with placebo treatment (both p > 0.80) and were not influenced by SAMS status (both p > 0.35 in the multiple linear regression model). By contrast, the Δ in vitamin D with placebo treatment was inversely associated with Δ in PSS (Pearson coefficient = −0.21; p = 0.03); this was not observed with statin treatment (p=0.61) and was influenced by SAMS status (p < 0.01; Fig. 4) as it was marginally significant in patients without validated SAMS (p = 0.07) but insignificant in those with SAMS (p=0.25).
Fig. 3. Creatine kinase levels and vitamin D.

Relationship between the chance in creatine kinase with simvastatin treatment and post-simvastatin vitamin D levels, graphed as data points in individual patients with confirmed SAMS (black circles) and without SAMS (white triangles). There was no influence of SAMS so the regression line represents all data in the sample.
Fig. 4. Pain severity and vitamin D.
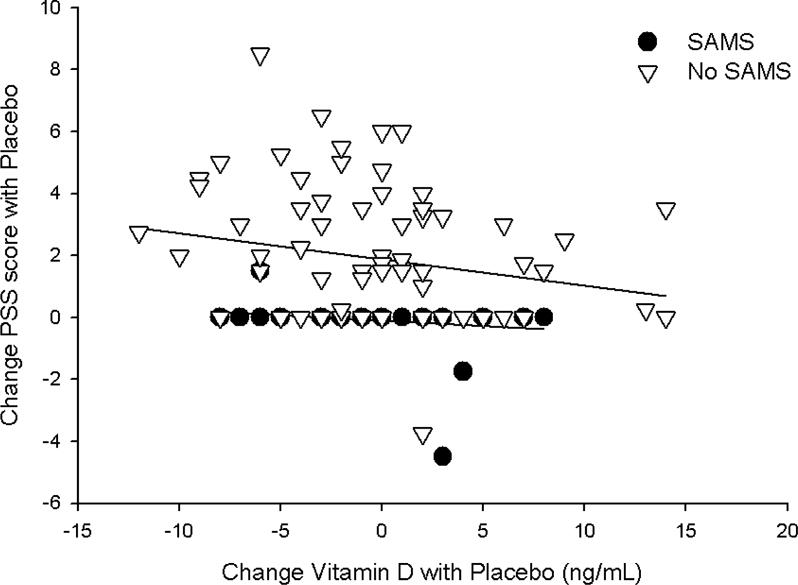
Relationship between the change in pain severity score (PSS) and change in vitamin D with placebo treatment, graphed as data points in individual patients with confirmed SAMS (black circles) and without SAMS (white triangles). There was a significant influence of SAMS (p < 0.01) so regression lines are graphed for each group separately.
DISCUSSION
Low vitamin D levels are a potential contributor to developing SAMS, but results from previous investigations have been equivocal. We performed a retrospective analysis of vitamin D levels before and after statin and placebo treatment in 120 patients in whom SAMS were validated using a rigorous double-blind, cross-over study design. There was no impact of baseline vitamin D levels or changes in vitamin D with statin therapy on the development of SAMS, nor did clinical vitamin D deficiency or insufficiency differentiate the development of SAMS. However, there were associations between baseline and post-treatment vitamin D levels and Δ in CK with statin therapy, as well as Δ in vitamin D levels and pain with placebo. This suggests that low vitamin D may play a role in both statin-associated muscle damage as well as non-specific muscle pain, potentially explaining the variable results with vitamin D in past studies.
Molecular mechanisms of vitamin D action on muscle tissue include genomic and non-genomic effects via receptors present in and on muscle cells (17, 18), and vitamin D deficiency alone can cause skeletal muscle myopathy (19) as well as decreased muscle strength (20, 21). Cholesterol is used to synthesize 7-dehydrocholesterol (7-DHC), the precursor to vitamin D3 (cholecalciferol), but studies to date have shown that statin therapy either has no effect on vitamin D (22, 23), or, conversely, increases vitamin D levels in asymptomatic adults (24, 25). Despite these latter findings, several studies and systematic reviews (7–9, 26, 27) have linked low vitamin D levels to SAMS, and consequently vitamin D supplementation to mitigation of muscle symptoms (10, 28), although other investigations have not supported this association (11, 12, 29). In the current report, we found no association between vitamin D levels, pre- and post-statin therapy, or clinical vitamin D deficiency and insufficiency, on the development of SAMS. These results persisted despite controlling for seasonal variations in vitamin D, which were significantly impacted by statin therapy but not placebo treatment.
We did observe that, regardless of SAMS, patients with lower vitamin D levels pre- or post-statin treatment exhibited greater increases in CK with statin therapy. Moreover, patients with greater reductions in vitamin D during placebo treatment had greater increases in self-reported pain severity; this relationship was observed in patients without SAMS but not with SAMS (likely because patients with confirmed SAMS by study criteria did not exhibit marked changes in muscle pain on placebo). These two associations support that low vitamin D levels are related to transient increases in muscle pain and damage, and that the damage may be exacerbated by statin therapy. This potential muscle injury was not specific to SAMS because it occurred with SIMVA treatment even in those not confirmed to have SAMS. It is possible that the 8-week duration of SIMVA treatment was too short for these subjects to develop SAMS and that a study with a longer duration of statin therapy in subjects with low vitamin D levels would produce SAMS in these subjects since they do show CK evidence of muscle injury. Consequently, these results cannot dismiss low vitamin D as a factor in SAMS and suggest that low vitamin D may indeed be a potential mechanism contributing to SAMS.
Alternatively, the observation that reductions in vitamin D were related to increased muscle pain with placebo could indicate that low vitamin D is related to nonspecific muscle pain. Since research from our laboratory as well as others (1, 13, 14, 30) suggests that only 30–50% of patients with self-reported SAMS actually experience muscle pain due to statins, it is possible that vitamin D deficiency may contribute to nonspecific muscle complaints rather than true SAMS for some patients. This needs to be confirmed in a larger trial, but could explain the variable findings on vitamin D status and the development of SAMS as well as vitamin D treatment for mitigation of SAMS (10).
There are limitations to the current study. As noted, the treatment duration of SIMVA and placebo was only 8 weeks each, and thus results may differ with longer statin treatment. The median time for the development of SAMS in this study as well as previous studies is approximately 1 month (1, 13, 31), but it is possible that longer term treatment would have unmasked a relationship between development of SAMS, changes in CK, and vitamin D levels. Moreover, we cannot make conclusions about associations between vitamin D and SAMS with other doses and types of statin therapy, as we only administered SIMVA 20 mg daily. Creatine kinase is sensitive to fluctuations in physical activity, but the definition of muscle symptoms in the current study attempted to limit those fluctuations by defining drug-related muscle symptoms as those unassociated with recent physical activity and measuring creatine kinase only after symptoms had persisted for a week (unless intolerable). In this cohort, patients with confirmed SAMS had higher CK at baseline (13), and changes in CK with simvastatin distinguished patients with confirmed SAMS from those with non-specific muscle pain (32). Therefore, we do not believe that physical activity patterns unduly influenced changes in CK, but caution is still warranted because CK is also influenced by factors such as age, sex, race and concomitant medications and cannot be systematically used to confirm SAMS. Only only ~25% of patients in the study were classified as vitamin D deficient, and thus enrolling only patients with very low levels of vitamin D deficiency may have produced a stronger relationship between very low vitamin D and SAMS. Finally, we did not conduct a supplementation trial of vitamin D in the current investigation as it was outside of the scope of the original funded trial design. The strengths of the current study include measuring vitamin D levels in patients before and after statin and placebo therapy, and confirming SAMS with a cross-over, placebo-controlled study design. This confirmation protocol yielded a similar percent of patients testing positive for SAMS as a recent clinical trial utilizing an almost identical design (14), but nonetheless the lack of sensitive biomarkers for SAMS still limits conclusive diagnostic capabilities (33).
There was no impact of baseline vitamin D levels or Δ in vitamin D with statin therapy on the development of SAMS, nor did clinical vitamin D deficiency or insufficiency differentiate the development of SAMS. Instead, associations between vitamin D levels and Δ in CK and pain severity with both statin and placebo therapy suggest that low vitamin D levels are related to transient increases in muscle pain and damage, and that the damage may be exacerbated by statin therapy. Low vitamin D could thus be a mechanism for both SAMS and non-specific muscle pain in patients on statins, but this relationship needs to be clarified in a large-scale clinical trial of vitamin D, statin treatment and SAMS.
Highlights.
We assessed associations between Vitamin D and development of statin-associated muscle symptoms (SAMS)
Baseline Vitamin D and changes in Vitamin D with simvastatin did not predict SAMS
Clinical Vitamin D deficiency and insufficiency did not predict SAMS
Low Vitamin D was associated with transient increases in creatine kinase and pain
Future studies are needed to clarify the relationship between Vitamin D and SAMS
Acknowledgments
The authors are grateful for the support of the Data Safety Monitoring Board: Ira Ockene, M.D., JoAnne Foody, M.D., and Pamela Hartigan, Ph.D.
Dr. Thompson has received research support from Genomas, Roche, Sanolfi, Regeneron, Esperion, Amarin and Pfizer; has served as a consultant for Amgen, Regeneron, Merck, Genomas, Runners World, Sanolfi, Esperion, and Amarin; has received speaker honoraria from Merck, Astra Zenica, Kowa, and Amarin; owns stock in Abbvie, Abbott Labs, General Electric, J&J; and has provided expert legal testimony on exercise-related cardiac events and statin myopathy. Dr. Taylor served on the Pharmacovigilance Monitoring Board for Amgen, Inc. and has received research support from Regeneron.
Financial support
Coenzyme Q10 in Statin Myopathy study was funded by NCCAM grant 1RC1AT005836 (P. Thompson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration
Conflict of interest
Mrs. Lorson and Dr. White do not have any conflicts of interest or financial disclosures.
Author contributions
All authors made substantial contributions to conception or design of the work and/or acquisition, analysis, or interpretation of data for the work as well as drafting, revising and reviewing the manuscript. In addition, Drs. Taylor and Thompson had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 3.Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, et al. Statin intolerance – an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittman DG, Chen W, Bowlin SJ, Foody JM. Adherence to statins, subsequent healthcare costs, and cardiovascular hospitalizations. Am J Cardiol. 2011;107:1662–6. doi: 10.1016/j.amjcard.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 5.Franklin JM, Krumme AA, Tong AY, Shrank WH, Matlin OS, Brennan TA, et al. Association between trajectories of statin adherence and subsequent cardiovascular events. Pharmacoepidemiol Drug Saf. 2015;24:1105–13. doi: 10.1002/pds.3787. [DOI] [PubMed] [Google Scholar]
- 6.Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7:472–83. doi: 10.1016/j.jacl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Morioka TY, Lee AJ, Bertisch S, Buettner C. Vitamin D status modifies the association between statin use and musculoskeletal pain: a population based study. Atherosclerosis. 2015;238:77–82. doi: 10.1016/j.atherosclerosis.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalska-Kasiczak M, Sahebkar A, Mikhailidis DP, Rysz J, Muntner P, Toth PP, et al. Analysis of vitamin D levels in patients with and without statin-associated myalgia – a systematic review and meta-analysis of 7 studies with 2420 patients. Int J Cardiol. 2015;178:111–6. doi: 10.1016/j.ijcard.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 9.Mergenhagen K, Ott M, Heckman K, Rubin LM, Kellick K. Low vitamin D as a risk factor for the development of myalgia in patients taking high-dose simvastatin: a retrospective review. Clin Ther. 2014;36:770–7. doi: 10.1016/j.clinthera.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Khayznikov M, Hemachrandra K, Pandit R, Kumar A, Wang P, Glueck CJ. Statin Intolerance Because of Myalgia, Myositis, Myopathy, or Myonecrosis Can in Most Cases be Safely Resolved by Vitamin D Supplementation. N Am J Med Sci. 2015;7:86–93. doi: 10.4103/1947-2714.153919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen A, Lev E, Iakobishvilli Z, Porter A, Brosh D, Hasdai D, et al. Low plasma vitamin D levels and muscle-related adverse effects in statin users. Isr Med Assoc J. 2014;16:42–5. [PubMed] [Google Scholar]
- 12.Kurnik D, Hochman I, Vesterman-Landes J, Kenig T, Katzir I, Lomnicky Y, et al. Muscle pain and serum creatine kinase are not associated with low serum 25(OH) vitamin D levels in patients receiving statins. Clin Endocrinol (Oxf) 2012;77:36–41. doi: 10.1111/j.1365-2265.2011.04321.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor BA, Lorson L, White CM, Thompson PD. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis. 2015;238:329–35. doi: 10.1016/j.atherosclerosis.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nissen SE, Stroes E, Dent-Acosta RE, Rosenson RS, Lehman SJ, Sattar N, et al. Efficacy and Tolerability of Evolocumab vs Ezetimibe in Patients With Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. JAMA. 2016;315:1580–90. doi: 10.1001/jama.2016.3608. [DOI] [PubMed] [Google Scholar]
- 15.Parker BA, Gregory SM, Lorson L, Polk D, White CM, Thompson PD. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187–93. doi: 10.1016/j.jacl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 17.De Boland AR, Boland RL. Non-genomic signal transduction pathway of vitamin D in muscle. Cell Signal. 1994;6:717–24. doi: 10.1016/0898-6568(94)00042-5. [DOI] [PubMed] [Google Scholar]
- 18.Boland RL. VDR activation of intracellular signaling pathways in skeletal muscle. Mol Cell Endocrinol. 2011;347:11–6. doi: 10.1016/j.mce.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Ziambaras K, Dagogo-Jack S. Reversible muscle weakness in patients with vitamin D deficiency. West J Med. 1997;167:435–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Mastaglia SR, Seijo M, Muzio D, Somoza J, Nunez M, Oliveri B. Effect of vitamin D nutritional status on muscle function and strength in healthy women aged over sixty-five years. J Nutr Health Aging. 2011;15:349–54. doi: 10.1007/s12603-010-0287-3. [DOI] [PubMed] [Google Scholar]
- 21.Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ, 3rd, Amin S. Is vitamin D a determinant of muscle mass and strength? J Bone Miner Res. 2011;26:2860–71. doi: 10.1002/jbmr.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail F, Corder CN, Epstein S, Barbi G, Thomas S. Effects of pravastatin and cholestyramine on circulating levels of parathyroid hormone and vitamin D metabolites. Clin Ther. 1990;12:427–30. [PubMed] [Google Scholar]
- 23.Rejnmark L, Vestergaard P, Heickendorff L, Mosekilde L. Simvastatin does not affect vitamin d status, but low vitamin d levels are associated with dyslipidemia: results from a randomised, controlled trial. Int J Endocrinol. 2010;2010:957174. doi: 10.1155/2010/957174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ertugrul DT, Yavuz B, Cil H, Ata N, Akin KO, Kucukazman M, et al. STATIN-D study: comparison of the influences of rosuvastatin and fluvastatin treatment on the levels of 25 hydroxyvitamin D. Cardiovasc Ther. 2011;29:146–52. doi: 10.1111/j.1755-5922.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- 25.Yavuz B, Ertugrul DT, Cil H, Ata N, Akin KO, Yalcin AA, et al. Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins? Cardiovasc Drugs Ther. 2009;23:295–9. doi: 10.1007/s10557-009-6181-8. [DOI] [PubMed] [Google Scholar]
- 26.Pereda CA, Nishishinya MB. Is there really a relationship between serum vitamin D (25OHD) levels and the musculoskeletal pain associated with statin intake? A systematic review. Reumatol Clin. 2016;16:30001–8. doi: 10.1016/j.reuma.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Ovesjo ML, Skilving I, Bergman P, Rane A, Ekstrom L, Bjorkhem-Bergman L. Low Vitamin D Levels and Genetic Polymorphism in the Vitamin D Receptor are Associated with Increased Risk of Statin-Induced Myopathy. Basic Clin Pharmacol Toxicol. 2016;118:214–8. doi: 10.1111/bcpt.12482. [DOI] [PubMed] [Google Scholar]
- 28.Jetty V, Glueck CJ, Wang P, Shah P, Prince M, Lee K, et al. Safety of 50,000–100,000 Units of Vitamin D3/Week in Vitamin D-Deficient, Hypercholesterolemic Patients with Reversible Statin Intolerance. N Am J Med Sci. 2016;8:156–62. doi: 10.4103/1947-2714.179133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riphagen IJ, van der Veer E, Muskiet FA, DeJongste MJ. Myopathy during statin therapy in the daily practice of an outpatient cardiology clinic: prevalence, predictors and relation with vitamin D. Curr Med Res Opin. 2012;28:1247–52. doi: 10.1185/03007995.2012.702102. [DOI] [PubMed] [Google Scholar]
- 30.Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23:1182–6. doi: 10.1007/s11606-008-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cham S, Evans MA, Denenberg JO, Golomb BA. Statin-associated muscle-related adverse effects: a case series of 354 patients. Pharmacotherapy. 2010;30:541–53. doi: 10.1592/phco.30.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor BA, Panza G, Thompson PD. Increased creatine kinase with statin treatment may identify statin-associated muscle symptoms. Int J Cardiol. 2016;209:12–3. doi: 10.1016/j.ijcard.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muntean DM, Thompson PD, Catapano AL, Stasiolek M, Fabis J, Muntner P, et al. Statin-associated myopathy and the quest for biomarkers: can we effectively predict statin-associated muscle symptoms? Drug Discov Today. 2016;16:30321. doi: 10.1016/j.drudis.2016.09.001. [DOI] [PubMed] [Google Scholar]
