Statins have immunomodulating properties that may increase the risk of varicella-zoster virus reactivation. We found that older patients treated with statins were at an increased risk of developing herpes zoster relative to individuals who were not prescribed to these drugs.
Keywords: herpes zoster, statins, drug safety, population-based, elderly
Abstract
Background. Statins are widely used lipid-lowering drugs with immunomodulatory properties that may favor reactivation of latent varicella-zoster virus infection. However, whether statins increase the risk of herpes zoster is unknown.
Methods. We conducted a population-based retrospective cohort study of Ontario residents aged ≥66 years between 1 April 1997 and 31 March 2010 to examine the association between statin use and incidence of herpes zoster. We used propensity score matching to ensure similarity between users and nonusers of statins, and Cox proportional hazard models to assess differences in outcomes between study groups. To test the specificity of our findings, we examined the association between statin exposure and knee arthroplasty.
Results. During the 13-year study period, we matched 494 651 individuals treated with a statin to an equal number of untreated individuals. In the main analysis, the rate of herpes zoster was higher among users of statins relative to nonusers of these drugs (13.25 vs 11.71 per 1000 person-years, respectively; hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.10–1.17). The attributable fraction of exposed individuals was 11.6%. In a prespecified analysis, we found a similar risk of herpes zoster among statin users in the subgroup of patients with diabetes (HR, 1.18; 95% CI, 1.09–1.27). As expected, we found no association between statin use and knee arthroplasty (HR, 1.04; 95% CI, .99–1.09).
Conclusions. Among older patients, treatment with statins is associated with a small but significantly increased risk of herpes zoster.
(See the editorial commentary by Pirmohamed on pages 357–8.)
Herpes zoster, a common illness caused by reactivation of latent varicella-zoster virus (VZV) infection, is associated with significant morbidity and healthcare costs [1–4]. The incidence and severity of herpes zoster and its debilitating sequelae increase with age, with >60% of cases occurring in individuals aged ≥50 years [5]. Although an age-related decline in VZV-specific cell-mediated immunity is the main determinant of viral reactivation, several studies have noted an increased incidence of herpes zoster in otherwise healthy older individuals independent of changes in the prevalence of immunosuppression, access to healthcare, or the introduction of varicella vaccination [5–8]. Because of the substantial burden imparted by herpes zoster and its rising incidence among older individuals, elucidation of risk factors associated with this illness has gained importance in recent years [2].
Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, generically referred to as statins, are among the most widely prescribed medications worldwide, with 25% of American adults aged ≥45 years treated with a statin in 2008 [9]. Along with being potent lipid-lowering agents, statins have immunomodulating properties that may increase the risk of VZV reactivation. Specifically, statin-mediated inhibition of HMG-CoA reductase decreases the synthesis of isoprenoid pyrophosphates, required for the activation of Ras-related GTPases [10, 11]. Because these proteins are involved in antigen endocytosis, immunologic synapse formation, and costimulatory molecule expression, T-cell activation and proliferation are impaired [10, 11]. In addition, statins can increase the number of CD4+CD25+ regulatory T cells, the accumulation of which may contribute to age-related immunosenescence and reactivation of latent infectious diseases [12, 13]. Finally, statin-induced promotion of Th2-cell differentiation could suppress Th1-type immune responses involved in controlling VZV replication [10, 14–16].
However, to our knowledge, no studies have examined whether statin therapy is associated with an increased risk of herpes zoster. We therefore compared the risk of herpes zoster in older patients receiving statins with that of nonusers of these drugs. We also examined the risk of herpes zoster in the subset of patients with diabetes, as these patients may have reduced VCV–specific cell-mediated immunity relative to healthy individuals and are therefore at higher risk of herpes zoster [17–21]. We speculated that by virtue of their immunomodulatory properties, statins would be associated with an increased incidence of herpes zoster.
METHODS
Setting
We conducted a population-based retrospective cohort study of all Ontario residents aged ≥66 years between 1 April 1997 and 31 March 2010, using administrative healthcare data and propensity score matching to ensure comparability in measured confounders between users and nonusers of statins. These individuals had universal access to physician services, hospital care, and prescription drug coverage.
Data Sources
We determined medication exposure using the Ontario Drug Benefit Database, which contains comprehensive records of prescription drugs dispensed to Ontario residents aged ≥65 years. To avoid incomplete medication records, we excluded patients during their first year of eligibility for prescription drug coverage (age 65). We obtained hospitalization and emergency department data from the Canadian Institute for Health Information's Discharge Abstract Database and National Ambulatory Care Reporting System, respectively. These databases contain detailed clinical information regarding all hospital admissions and emergency department visits in Ontario. We used the Ontario Health Insurance Plan database to identify claims for physician services, and the Ontario Diabetes Database, a validated database of individuals with diabetes in Ontario, to define diabetes diagnosis [22]. We obtained basic demographic data and date of death from the Registered Persons Database, a registry of all Ontario residents eligible for health insurance. These databases were linked in an anonymous fashion using encrypted health card numbers, and are routinely used to explore postmarketing drug safety issues [23, 24].
Study Subjects
We identified 2 groups of individuals for comparison. The exposed group consisted of individuals who commenced treatment with any 1 of the 7 statins available in Ontario during the study period (atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), and we defined the index date as the date of a patient's first prescription for a statin. To restrict our analysis to new users of these drugs, we excluded individuals who had received a prescription for any statin in the year preceding the index date. We defined continuous use of a statin as refills of the drug within twice the duration of the preceding prescription. We retained individuals who switched among the available statins, but censored those who discontinued treatment, defined by the date of their final prescription plus the last prescription's days' supply.
For each statin-exposed individual, we identified 1 untreated individual using propensity score matching according to the following algorithm [25]. First, we derived propensity scores for statin therapy for each patient by using a nonparsimonious logistic regression model that included statin exposure as the dependent variable and an extensive list of variables related to either the prescription of statins or the risk of herpes zoster (Supplementary Appendix). We then used a greedy matching algorithm to pair each patient receiving a statin with an untreated individual based on the logit of the propensity score (within 0.2 standard deviations), age at index date (within 2 years), sex, year of cohort entry, and presence or absence of diabetes. In both groups, we excluded all individuals who had been diagnosed with herpes zoster in the 5 years preceding the index date.
Outcomes
The primary outcome of the study was a new diagnosis of herpes zoster, defined as any one of a physician visit, emergency department visit or hospital admission for herpes zoster (International Classification of Diseases [ICD], Ninth and Tenth revisions, codes 053 and B02, respectively), or the receipt of a prescription for either valaciclovir or famciclovir, which are restricted by the Ontario Drug Benefit program solely for the treatment of herpes zoster. We considered only the first episode of herpes zoster as a study outcome in patients with multiple episodes during the study period. We followed each person in the cohort for up to 2 years from their index date until the occurrence of the outcome, death, statin discontinuation, or end of the study period (31 March 2011), whichever occurred first. To test the specificity of our findings, we examined the association between statin use and admissions for knee arthroplasty. Because there is no plausible reason why statin therapy should be associated with knee arthroplasty, we reasoned that a null association with this outcome would enhance causal inference.
Statistical Analysis
We computed standardized differences to examine intergroup balance in the distribution of baseline variables. Standardized differences of <0.1 indicate good balance between groups for a given covariate [26]. We conducted time-to-event analyses using Cox proportional hazards regression to examine the association of statins with herpes zoster reactivation, using unexposed individuals as the reference group. To test the robustness of our findings to the possibility of an induction period for statin exposure, we replicated our analysis including only those individuals who filled >1 prescription for a statin. In a prespecified analysis, we also explored the association between statin use and herpes zoster in the subgroup of patients with diabetes, because these patients may be at higher risk for herpes zoster and more likely to be prescribed a statin [17–21]. Finally, we stratified statin doses into 1 of 3 categories based on published estimates of expected reductions in low-density lipoprotein cholesterol from baseline [27, 28]. We then conducted a dose-response assessment by considering low (atorvastatin <20 mg, rosuvastatin <10 mg, cerivastatin <0.3 mg, simvastatin <80 mg, fluvastatin at all doses, pravastatin at all doses, lovastatin at all doses), medium (atorvastatin 20 to <80 mg, rosuvastatin 10 to <40 mg, simvastatin ≥80 mg, cerivastatin 0.3 to 0.4 mg), and high (atorvastatin ≥80 mg, rosuvastatin ≥40 mg, cerivastatin ≥0.4 mg) doses of statins as time-dependent covariates in the analyses, using low-dose treatment as the reference. We verified the proportional hazards assumption by testing the statistical significance of a time-dependent treatment variable and by visually inspecting the estimated log(-log) survival curves. We calculated the attributable fraction among exposed individuals, defined as the proportion of herpes zoster episodes in individuals treated with statins that might have been avoided had exposure to statins not occurred [29]. All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
Ethics
This study was approved by the Research Ethics Board of the Sunnybrook Health Sciences Centre, Toronto.
RESULTS
During the 13-year accrual period, we identified 494 651 older individuals who received a prescription for a statin and an equal number of matched unexposed subjects. Patients treated with a statin were followed for a median of 696 days (interquartile range, 100–731 days), and untreated individuals were followed for a median of 731 days (interquartile range, 731–731 days). Collectively, individuals in the cohort provided a total of 1 553 288 person-years of follow-up. Following propensity score matching, users and nonusers of statins were highly similar with respect to demographics, medical illnesses, and concomitant medications (Table 1).
Table 1.
Baseline Characteristics
| Variable | Statin Users (n = 494 651) | Matched Control Group (n = 494 651) | Standardized Difference |
|---|---|---|---|
| Age, y, median (IQR) | 73 (69–78) | 73 (69–78) | 0.01 |
| 66–74 | 283 288 (57.3%) | 281 121 (56.8%) | 0.01 |
| 75–84 | 175 073 (35.4%) | 176 598 (35.7%) | 0.01 |
| ≥85 | 36 290 (7.3%) | 36 932 (7.5%) | 0.01 |
| Female sex | 272 921 (55.2%) | 272 921 (55.2%) | 0.00 |
| Charlson Comorbidity Index | |||
| No hospitalization | 305 282 (61.7%) | 310 836 (62.8%) | 0.02 |
| 0 | 77 853 (15.7%) | 81 922 (16.6%) | 0.02 |
| 1 | 45 614 (9.2%) | 43 869 (8.9%) | 0.01 |
| ≥2 | 65 902 (13.3%) | 58 024 (11.7%) | 0.05 |
| History of chronic kidney disease (1 y) | 12 941 (2.6%) | 11 247 (2.3%) | 0.02 |
| Residence in a long-term care facility | 12 386 (2.5%) | 10 368 (2.1%) | 0.03 |
| No. of prescription drugs in previous year, median (IQR) | 5 (3–9) | 5 (2–9) | 0.01 |
| Medication use in previous year | |||
| Oral corticosteroids | 21 986 (4.4%) | 22 061 (4.5%) | 0.00 |
| Immune modulating drugs | 3914 (0.8%) | 4278 (0.9%) | 0.01 |
| Income quintile | |||
| 1 (lowest) | 100 848 (20.4%) | 97 403 (19.7%) | 0.02 |
| 2 | 105 913 (21.4%) | 105 448 (21.3%) | 0.00 |
| 3 | 97 672 (19.7%) | 98 614 (19.9%) | 0.01 |
| 4 | 92 618 (18.7%) | 95 226 (19.3%) | 0.01 |
| 5 | 95 680 (19.3%) | 95 995 (19.4%) | 0.00 |
| Medical conditions and procedures in previous 5 y | |||
| Myocardial infarction | 28 720 (5.8%) | 22 072 (4.5%) | 0.06 |
| Angina | 38 053 (7.7%) | 31 345 (6.3%) | 0.05 |
| Diabetes | 99 982 (20.2%) | 99 982 (20.2%) | 0.00 |
| Hypertension | 326 705 (66.0%) | 333 370 (67.4%) | 0.03 |
| Stroke or transient ischemic attack | 25 674 (5.2%) | 25 071 (5.1%) | 0.01 |
| Systemic malignancy | 23 332 (4.1%) | 21 432 (4.3%) | 0.01 |
| Ulcerative colitis | 3611 (0.7%) | 3733 (0.8%) | 0.00 |
| Crohn's disease | 2839 (0.6%) | 2923 (0.6%) | 0.00 |
| Systemic lupus erythematosus | 11 412 (2.3%) | 11 648 (2.4%) | 0.00 |
| Rheumatoid arthritis | 25 674 (5.2%) | 25 071 (5.1%) | 0.00 |
| HIV infection | 81 (0.0%) | 126 (0.0%) | 0.01 |
| Coronary artery bypass grafting | 7292 (1.5%) | 5874 (1.2%) | 0.03 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
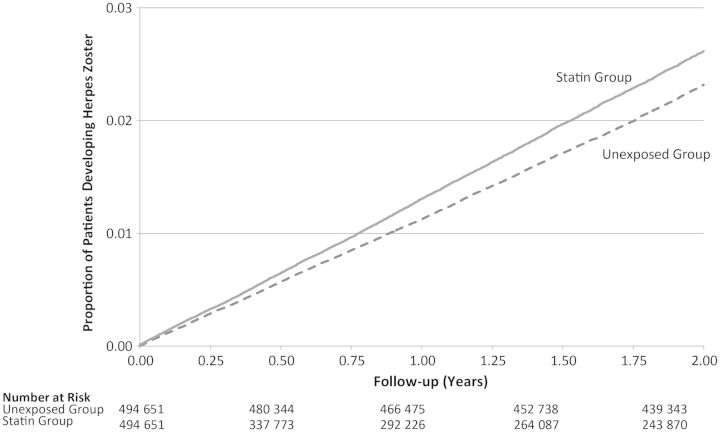
In the main analysis, the primary outcome of herpes zoster occurred in a total of 19 143 individuals, with a higher rate among patients receiving a statin relative to nonusers of these drugs (13.25 vs 11.71 per 1000 person-years, respectively; hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.10–1.17; Figure 1). These results were unchanged following replication of our analyses among only those individuals who filled >1 prescription for a statin. We found a similar risk of herpes zoster among statin users in the subgroup of patients with diabetes (HR, 1.18; 95% CI, 1.09–1.27; Table 2).
Figure 1.
Kaplan-Meier curves for herpes zoster, by statin use.
Table 2.
Association Between Statin Use and Herpes Zoster
| Outcome | No. Patients per Group | Rate in Individuals Prescribed a Statin (per 1000 Person-years) | Rate in Individuals Not Prescribed a Statin (per 1000 Person-years) | Hazard Ratio (95% Confidence Interval)a |
|---|---|---|---|---|
| Primary outcome | ||||
| Entire cohort | 494 651 | 13.25 | 11.71 | 1.13 (1.10–1.17) |
| Diabetic subgroup | 99 982 | 13.17 | 11.07 | 1.18 (1.09–1.27) |
| Tracer outcome | ||||
| Knee arthroplasty | 494 651 | 6.70 | 6.32 | 1.04 (.99–1.09) |
a Reference group is individuals who were not prescribed a statin.
In the dose-response analysis, we found no appreciable difference in the risk of herpes zoster with moderate (HR, 1.05; 95% CI, 1.00–1.10) or high doses (HR, 1.03; 85% CI, .90–1.19) of statins relative to low doses of these drugs. However, these results are limited by the fact that <1 in 10 patients experienced an increase in their statin dose from baseline (Supplementary Tables 1 and 2). As expected, we found no association between statin use and knee arthroplasty (HR, 1.04; 95% CI, .99–1.09; Table 2).
The attributable fraction of exposed individuals was 11.6%, suggesting that slightly more than 1 in 10 episodes of herpes zoster among older individuals taking statins was attributable to use of these drugs, and as such could be avoided if the drugs were not prescribed.
DISCUSSION
In this population-based study of nearly 1 million older individuals, we found that statin-treated patients were at an increased risk of developing herpes zoster relative to individuals who were not prescribed these drugs. We observed similar results in a subgroup of patients with diabetes. In contrast, we found no association between statin use and knee arthroplasty. Our study provides the first empirical evidence of an association between statins and herpes zoster, and suggests that the widespread use of these drugs may be an important factor accounting for the increased incidence of this disease among otherwise immunocompetent older individuals [5–8].
Our findings have important implications for public health. Given the large number of older individuals who are simultaneously at risk of developing herpes zoster and receiving a statin, even the small increase in risk observed in this study could translate into a large number of excess cases of herpes zoster at the population level. Assuming an exposure prevalence of 25% in American adults >50 years of age, a similar risk increase, and 628 000 cases of herpes zoster each year in this population, we estimate that nearly 20 000 of these episodes could be attributable to statin use [5, 9]. Although generally not life-threatening, herpes zoster and its sequelae are associated with considerable morbidity, reduced quality of life, and cost to the healthcare system, estimated at more than $1 billion annually in the United States alone [30]. Consequently, strategies aimed at reducing the risk of herpes zoster are essential for mitigating the burden associated with this disease. While our study should not deter clinicians from prescribing statins for patients with known coronary heart disease, our findings support the thoughtful reappraisal of statin therapy for otherwise healthy individuals who are at low risk for cardiovascular disease [31, 32]. In these patients, the benefits ascribed to statin therapy may be outweighed by the risk of serious adverse effects associated with these drugs [33, 34]. For patients in whom statin therapy is required, our findings suggest that provision of the herpes zoster vaccine should be considered.
Some limitations of our work merit emphasis. As with all observational studies, it is possible that our findings are biased by unmeasured confounders or intergroup differences in the baseline risk of herpes zoster. However, this is unlikely because both groups were well matched with respect to baseline characteristics, such that any residual differences were negligible and unlikely to account for our findings. In addition, it is difficult to envision an unmeasured variable that would be strongly associated with herpes zoster and distributed differentially among the 2 groups to an extent that could sufficiently compromise the validity of our findings. Moreover, we found no differential risk of knee arthroplasty, highlighting the specificity of our findings. We had no access to serum cholesterol levels, which were correlated with herpes zoster in a small study of cardiac transplant recipients [35], or measures of adherence to statin drugs. We could not capture prescriptions for statins initiated in hospitalized patients. However, exposure misclassification is unlikely as most patients fill prescriptions within 1 week of their discharge from Ontario hospitals [36]. Although individuals treated with statins may have more medical encounters than untreated patients, differential outcome ascertainment is unlikely given that 95% of older adults with herpes zoster seek medical attention [37]. Although the accuracy of coding for herpes zoster is not known in our particular setting, the positive predictive value of the ICD-9 code (053) for herpes zoster was 93% in an earlier study [38]. Finally, we could not reliably examine the association between statin dose and risk of herpes zoster given the rarity of high-dose statin therapy in our cohort.
In summary, our study suggests that statins are associated with an increased risk of herpes zoster in older patients. Given the widespread use of statins and the substantial morbidity associated with herpes zoster, further research is needed to clarify the exact mechanisms through which statins promote VZV reactivation and whether our findings are generalizable to younger patients receiving these drugs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Brogan Inc, Ottawa, for use of their Drug Product and Therapeutic Class Database. We thank Diana Martins for her assistance with data analysis.
Author contributions. Study concept and design: T. A. (guarantor), S. S., T. G., D. N. J., M. M. M. Analysis and interpretation of data: T. A., S. S., T. G., D. N. J., M. M. M., H. Z. Acquisition of data: H. Z. Drafting of the manuscript: T. A. Critical revision of manuscript: T. A., S. S., T. G., D. N. J., M. M. M., H. Z. Administrative, technical, or material support: T. A., T. G., H. Z. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support. T. A. is supported by a postdoctoral fellowship from the Ontario HIV Treatment Network. This work was supported by research funds from the Ontario Drug Policy Research Network and by the Institute for Clinical Evaluative Sciences (ICES), which is funded by a grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Potential conflicts of interest. M. M. M. has been on advisory boards and received honoraria from AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Hoffman La Roche, Novartis, Novo Nordisk, and Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211–5. doi: 10.1136/bmj.39206.571042.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26–33. doi: 10.1016/s1473-3099(03)00857-0. [DOI] [PubMed] [Google Scholar]
- 3.Stein AN, Britt H, Harrison C, Conway EL, Cunningham A, MacIntyre CR. Herpes zoster burden of illness and health care resource utilization in the Australian population aged 50 years and older. Vaccine. 2009;27:520–9. doi: 10.1016/j.vaccine.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin RH, White R, O'Connor AB, Baser O, Hawkins K. Healthcare costs of acute and chronic pain associated with a diagnosis of herpes zoster. J Am Geriatr Soc. 2007;55:1168–75. doi: 10.1111/j.1532-5415.2007.01231.x. [DOI] [PubMed] [Google Scholar]
- 5.Yawn BP, Saddier P, Wolan PC, St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–9. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 6.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–40. doi: 10.1093/cid/ciq077. [DOI] [PubMed] [Google Scholar]
- 7.Brisson M, Edjmunds WJ, Law B, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127:305–14. doi: 10.1017/s0950268801005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clin Infect Dis. 2010;50:1000–5. doi: 10.1086/651078. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics. Hyattsville, MD: Author; 2011. Health, United States, 2010: with special feature on death and dying. [PubMed] [Google Scholar]
- 10.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nature Rev Immunol. 2006;6:358–70. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghittoni R, Lazzerini PE, Pasini FL, Baldari CT. T lymphocytes as targets of statins: molecular mechanisms and therapeutic perspectives. Inflamm Allergy Drug Targets. 2006;6:3–16. doi: 10.2174/187152807780077291. [DOI] [PubMed] [Google Scholar]
- 12.Mausner-Fainberg K, Luboshits G, Mor A, et al. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Artherosclerosis. 2008;197:829–39. doi: 10.1016/j.atherosclerosis.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annu Rev Immunol. 2009;27:551–89. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 14.Asanuma H, Sharp M, Maecker HT, Maino VC, Arvin AM. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–66. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins DE, Redman RL, Lam EM, Liu C, Lin I, Arvin AM. Interleukin (IL)-10, IL-12 and interferon gamma production in primary and memory immune responses to varicella-zoster virus. J Infect Dis. 1998;178:950–8. doi: 10.1086/515702. [DOI] [PubMed] [Google Scholar]
- 16.Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–44. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y. Comparison of varicella-zoster virus specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis. 2009;200:1606–10. doi: 10.1086/644646. [DOI] [PubMed] [Google Scholar]
- 18.Hata A, Kuniyoshi M, Ohkusa Y. Risk of herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39:537–44. doi: 10.1007/s15010-011-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463–9. doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Jih JS, Chen YJ, Lin MW, et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Derm Venereol. 2009;89:612–6. doi: 10.2340/00015555-0729. [DOI] [PubMed] [Google Scholar]
- 21.Heymann AD, Chodick G, Karpati T, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection. 2008;36:226–30. doi: 10.1007/s15010-007-6347-x. [DOI] [PubMed] [Google Scholar]
- 22.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–6. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 23.Juurlink DN, Gomes T, Lipscombe LL, Austin PC, Hux JE, Mamdani MM. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ. 2009;339:b2942. doi: 10.1136/bmj.b2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354:1352–61. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. An introduction to propensity score methods to reduce the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 27.Law MR, Wald JN, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plosker GL, Dunn CI, Figgitt DP. Cerivastatin: a review of its pharmacological properties and therapeutic efficacy in the management of hypercholesterolaemia. Drugs. 2000;60:1179–206. doi: 10.2165/00003495-200060050-00011. [DOI] [PubMed] [Google Scholar]
- 29.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White RW, Lenhart G, Singhal P, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics. 2009;27:781–92. doi: 10.2165/11317560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.de Lorgeril M, Salen P, Abramson J, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-Jupiter controversy: a critical reappraisal. Arch Intern Med. 2010;170:1032–36. doi: 10.1001/archinternmed.2010.184. [DOI] [PubMed] [Google Scholar]
- 32.Taylor F, Ward K, Moore THM, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011:CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women's Health Initiative. Arch Intern Med. 2012;172:144–52. doi: 10.1001/archinternmed.2011.625. [DOI] [PubMed] [Google Scholar]
- 34.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population base cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Pozo JL, van de Beek D, Mandrekar JN, et al. High serum cholesterol levels are associated with herpes zoster infection after heart transplantation. Clin Infect Dis. 2010;50:121–2. doi: 10.1086/649005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackevicius CA, Materson JM, Naglie G. Concordance between discharge prescriptions and insurance claims in post-myocardial infarction patients. Pharmacoepidemiol Drug Saf. 2007;16:207–15. doi: 10.1002/pds.1289. [DOI] [PubMed] [Google Scholar]
- 37.Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine. 2007;25:7598–604. doi: 10.1016/j.vaccine.2008.11.077. [DOI] [PubMed] [Google Scholar]
- 38.Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86:1146–53. doi: 10.4065/mcp.2011.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.