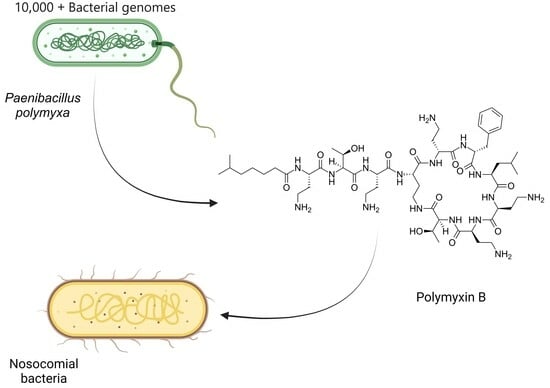
Nosocomial Bacteria Inhibition with Polymyxin B: In Silico Gene Mining and In Vitro Analysis
"> Figure 1
<p>(<b>A</b>). Estimated non-ribosomal peptide length (average) in select genera (purple) and average cationic residues per peptide (blue): <span class="html-italic">Paenibacillus</span> n = 602 (range of peptide length = 1–30), <span class="html-italic">Brevibacillus</span> n = 249 (range of peptide length = 1–33), <span class="html-italic">Streptomyces</span> n = 2592 (range of peptide length = 1–56), <span class="html-italic">Bacillus</span> n = 4840 (range of peptide length = 1–26), <span class="html-italic">Pseudomonas</span> n = 1758 (range of peptide length = 1–48), and <span class="html-italic">Burkholderia</span> n = 1915 (range of peptide length = 1–28). (<b>B</b>). Fraction of bacteria that contain essential predicted residues for PPPB. legend for circle graph (<b>C</b>). Total number of organisms with the biological potential of producing polymyxin (* commercially available). Range of peptide length (n) (<span class="html-italic">P. lentus</span> DSM 25539 (1–15), <span class="html-italic">P.</span> sp. IHB B 3084, <span class="html-italic">P. polymyxa</span> CR1 (2–14), <span class="html-italic">P. polymyxa</span> ZF129 (3–13) <span class="html-italic">P. polymyxa</span> J (3–14), <span class="html-italic">P. polymyxa</span> Sb3-1 (4–12)<span class="html-italic">, P. polymyxa</span> SQR-21, ATCC 15970, HY96-2 (4–13), <span class="html-italic">P. polymyxa</span> E681, YC0136, <span class="html-italic">P. peoriae</span> HS311 (4–14), <span class="html-italic">P</span>. sp. lzh-N1 (4–16), and <span class="html-italic">P</span>. sp. M-152 (4–18).</p> "> Figure 1 Cont.
<p>(<b>A</b>). Estimated non-ribosomal peptide length (average) in select genera (purple) and average cationic residues per peptide (blue): <span class="html-italic">Paenibacillus</span> n = 602 (range of peptide length = 1–30), <span class="html-italic">Brevibacillus</span> n = 249 (range of peptide length = 1–33), <span class="html-italic">Streptomyces</span> n = 2592 (range of peptide length = 1–56), <span class="html-italic">Bacillus</span> n = 4840 (range of peptide length = 1–26), <span class="html-italic">Pseudomonas</span> n = 1758 (range of peptide length = 1–48), and <span class="html-italic">Burkholderia</span> n = 1915 (range of peptide length = 1–28). (<b>B</b>). Fraction of bacteria that contain essential predicted residues for PPPB. legend for circle graph (<b>C</b>). Total number of organisms with the biological potential of producing polymyxin (* commercially available). Range of peptide length (n) (<span class="html-italic">P. lentus</span> DSM 25539 (1–15), <span class="html-italic">P.</span> sp. IHB B 3084, <span class="html-italic">P. polymyxa</span> CR1 (2–14), <span class="html-italic">P. polymyxa</span> ZF129 (3–13) <span class="html-italic">P. polymyxa</span> J (3–14), <span class="html-italic">P. polymyxa</span> Sb3-1 (4–12)<span class="html-italic">, P. polymyxa</span> SQR-21, ATCC 15970, HY96-2 (4–13), <span class="html-italic">P. polymyxa</span> E681, YC0136, <span class="html-italic">P. peoriae</span> HS311 (4–14), <span class="html-italic">P</span>. sp. lzh-N1 (4–16), and <span class="html-italic">P</span>. sp. M-152 (4–18).</p> "> Figure 2
<p>Media effect on <span class="html-italic">P. polymyxa</span> growth [ATCC-recommended media (M178, blue), tryptic soy broth (TSB, red), tryptic soy broth with starch (20 g/L) (TSB S20, green), tryptic soy broth with starch (40 g/L) (TSB S40, purple), Luria–Bertani broth (LB, orange), and yeast extract peptone dextrose (YPD, dark green)] (three trials in triplicate and error bars are SDs).</p> "> Figure 3
<p>Fragmented MS/MS peaks using TOF of 1203.3698 Da peak; y-axis on right shows absolute intensity.</p> "> Figure 4
<p>PMB inhibits the growth of several bacterial pathogens. The MBIC of PMB to each strain was determined as described in the Materials and Methods section. (<b>A</b>) The effect of PMB on three <span class="html-italic">P. aeruginosa</span> multidrug-resistant strains (MRSN 17849, MRSN 18560, and MRSN 2108. (<b>B</b>) The effect of PMB on the <span class="html-italic">K. pneumoniae</span> strain KP-UTI-2 and the <span class="html-italic">A. baumannii</span> strain AB-10. Bars indicate the means of three independent experiments. *, <span class="html-italic">p</span> < 0.05; ****, <span class="html-italic">p</span> < 0.0001; ns, not significant. Statistical significance (****) was determined using a two-way ANOVA with Tukey’s multiple comparison test. The growth of <span class="html-italic">P. aeruginosa</span> strains MRSN-17849 and MRSN-2108 was inhibited by 4 mg/mL (no CFU was recovered). Similarly, the <span class="html-italic">K. pneumoniae</span> strain KP-UTI-2 and the <span class="html-italic">A. baumannii</span> strain AB-10 were inhibited by 2 mg/mL. In the graphs, we included 4–5 CFUs for each point to conduct the statistical analysis.</p> ">
Abstract
:1. Introduction
2. Results
2.1. Genome Mining for Non-Ribosomal Polypeptides
2.2. Polymyxin B Biosynthetic Gene Clusters
2.3. Confirming PMB Competence
2.4. NRP Antibiotic Isolation, Purification, and Characterization
2.5. Tested Bacterial Strains Varied in Their Susceptibility to PMB
3. Discussion
4. Materials and Methods
4.1. Genome Mining
4.2. Culture Conditions
4.3. Polymyxin Extraction
4.3.1. Fermentation Broth Acid Precipitation
4.3.2. Resin Extractions
4.4. In Vitro Antibiotic Assay
4.5. PMB Isolation and Purification
4.6. MALDI TOF MS Analysis
4.7. Minimum Inhibitory Concentration
4.8. Assessing the Minimum Bactericidal Inhibitory Concentration (MBIC)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarzer, D.; Finking, R.; Marahiel, M.A. Nonribosomal peptides: From genes to products. Nat. Prod. Rep. 2003, 20, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Finking, R.; Marahiel, M.A. Biosynthesis of nonribosomal peptides. Annu. Rev. Microbiol. 2004, 58, 453–488. [Google Scholar] [CrossRef] [PubMed]
- Strieker, M.; Tanović, A.; Marahiel, M.A. Nonribosomal peptide synthetases: Structures and dynamics. Curr. Opin. Struct. Biol. 2010, 20, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Weber, T. In silico tools for the analysis of antibiotic biosynthetic pathways. Int. J. Med. Microbiol. 2014, 304, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Boddy, C.N. Bioinformatics tools for genome mining of polyketide and non-ribosomal peptides. J. Ind. Microbiol. Biotechnol. 2014, 41, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, N.D.; Moktali, V.; Medema, M.H. Bioinformatics approaches and software for detection of secondary metabolic gene clusters. Methods Mol. Biol. 2012, 944, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Leclere, V.; Weber, T.; Jacques, P.; Pupin, M. Bioinformatics Tools for the Discovery of New Nonribosomal Peptides. Methods Mol. Biol. 2016, 1401, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Vo, T.D.; Spahn, C.; Heilemann, M.; Bode, H.B. Microbial Cationic Peptides as a Natural Defense Mechanism against Insect Antimicrobial Peptides. ACS Chem. Biol. 2021, 16, 447–451. [Google Scholar] [CrossRef]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An update on the antibiotic of the 21st century. Expert. Rev. Anti Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhong, Z.; Zhang, W.P.; Qian, P.Y. Discovery of cationic nonribosomal peptides as Gram-negative antibiotics through global genome mining. Nat. Commun. 2018, 9, 3273. [Google Scholar] [CrossRef]
- Lohans, C.T.; van Belkum, M.J.; Cochrane, S.A.; Huang, Z.; Sit, C.S.; McMullen, L.M.; Vederas, J.C. Biochemical, structural, and genetic characterization of tridecaptin A(1), an antagonist of Campylobacter jejuni. Chembiochem 2014, 15, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Guo, Y.; Yousef, A.E. Draft genome sequence of Paenibacillus sp. strain OSY-SE, a bacterium producing the novel broad-spectrum lipopeptide antibiotic paenibacterin. J. Bacteriol. 2012, 194, 6306. [Google Scholar] [CrossRef]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Booysen, E.; Rautenbach, M.; Stander, M.A.; Dicks, L.M.T. Profiling the Production of Antimicrobial Secondary Metabolites by Xenorhabdus khoisanae J194 under Different Culturing Conditions. Front. Chem. 2021, 9, 626653. [Google Scholar] [CrossRef] [PubMed]
- Yoon, V.; Nodwell, J.R. Activating secondary metabolism with stress and chemicals. J. Ind. Microbiol. Biotechnol. 2014, 41, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, A.P.; Alferova, V.A.; Korshun, V.A. Chemical Elicitors of Antibiotic Biosynthesis in Actinomycetes. Microorganisms 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Tojo, S.; Tanaka, Y.; Ochi, K. Activation of Antibiotic Production in Bacillus spp. by Cumulative Drug Resistance Mutations. Antimicrob. Agents Chemother. 2015, 59, 7799–7804. [Google Scholar] [CrossRef]
- Shen, W.; Wang, D.; Wei, L.; Zhang, Y. Fungal elicitor-induced transcriptional changes of genes related to branched-chain amino acid metabolism in Streptomyces natalensis HW-2. Appl. Microbiol. Biotechnol. 2020, 104, 4471–4482. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, Z.; Yin, J.; Qiu, J. Enhanced Production of Polymyxin E in Paenibacillus polymyxa by Replacement of Glucose by Starch. Biomed. Res. Int. 2018, 2018, 1934309. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, X.; Cao, M.; Wang, C.; Zhang, C.; Lu, Z.; Lu, F. Genomics-Inspired Discovery of Three Antibacterial Active Metabolites, Aurantinins B, C, and D from Compost-Associated Bacillus subtilis fmb60. J. Agric. Food Chem. 2016, 64, 8811–8820. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.-A.; Fuchs, S.W.; Bode, H.B.; Grünewald, B. Low-Molecular-Weight Metabolites Secreted by Paenibacillus larvae as Potential Virulence Factors of American Foulbrood. Appl. Environ. Microbiol. 2014, 80, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Jangra, M.; Randhawa, H.K.; Kaur, M.; Srivastava, A.; Maurya, N.; Patil, P.P.; Jaswal, P.; Arora, A.; Patil, P.B.; Raje, M.; et al. Purification, Characterization and in vitro Evaluation of Polymyxin A From Paenibacillus dendritiformis: An Underexplored Member of the Polymyxin Family. Front. Microbiol. 2018, 9, 2864. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-C.; Shen, X.-B.; Ding, R.; Qian, C.-D.; Fang, H.-H.; Li, O. Isolation and partial characterization of antibiotics produced by Paenibacillus elgii B69. FEMS Microbiol. Lett. 2010, 310, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Vater, J.; Herfort, S.; Doellinger, J.; Weydmann, M.; Borriss, R.; Lasch, P. Genome Mining of the Lipopeptide Biosynthesis of Paenibacillus polymyxa E681 in Combination with Mass Spectrometry: Discovery of the Lipoheptapeptide Paenilipoheptin. ChemBioChem 2018, 19, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, C.; Orwa, J.; Van Schepdael, A.; Roets, E.; Hoogmartens, J. Characterization of polypeptide antibiotics of the polymyxin series by liquid chromatography electrospray ionization ion trap tandem mass spectrometry. J. Pept. Sci. 2002, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, F.; Snesrud, E.; Hall, L.; Mills, E.; Galac, M.; Stam, J.; Ong, A.; Maybank, R.; Kwak, Y.I.; Johnson, S.; et al. A panel of diverse Pseudomonas aeruginosa clinical isolates for research and development. JAC Antimicrob. Resist. 2021, 3, dlab179. [Google Scholar] [CrossRef] [PubMed]
- Stephany Navarro, A.H. Unpublished Results. 2024; manuscript in preparation. [Google Scholar]
- FDA Rationale for Polymyxin Breakpoints for Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter spp. Available online: https://www.fda.gov/drugs/development-resources/fda-rationale-polymyxin-breakpoints-enterobacterales-pseudomonas-aeruginosa-and-acinetobacter-spp (accessed on 3 August 2024).
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Newton, B.A. The properties and mode of action of the polymyxins. Bacteriol. Rev. 1956, 20, 14–27. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef] [PubMed]
- Ayoub Moubareck, C. Polymyxins and Bacterial Membranes: A Review of Antibacterial Activity and Mechanisms of Resistance. Membranes 2020, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Alterations in outer membrane permeability. Annu. Rev. Microbiol. 1984, 38, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Qin, W.; Lin, J.; Fang, S.; Qiu, J. Antibacterial Mechanisms of Polymyxin and Bacterial Resistance. BioMed Res. Int. 2015, 2015, 679109. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Song, J.; Velkov, T.; Wang, L.; Zhu, Y.; Li, J. Regulating polymyxin resistance in Gram-negative bacteria: Roles of two-component systems PhoPQ and PmrAB. Future Microbiol. 2020, 15, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 31 May 2024).
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Biemer, J.J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar]





| Organism | Metabolite |
|---|---|
| Paenibacillus polymyxa | (leu-thr-dab-dab) + (thr) + (dab-thr-dab-dab-dab) |
| (ala) + (ala) + (thr-val-ala-thr-asn-ala) | |
| (mal) + (pk-tyr-ala) + (ala) + (ser) + (ser-ile-ser) | |
| (asn) + (ala) + (ala) + (ala-gly) + (mal) + (ala-ala) + (val) | |
| (val-ala-gly dab-trp-dab-ala-ala-trp-glu) + (val-ile) + (ile) | |
| (pk-ala) + (thr-ser-orn-ala-ala) + (phe-ala-ala) | |
| (leu-thr-dab-dab) + (thr) + (dab-thr-dab-dab-dab) | |
| (val-ala-gly dab-trp-dab-ala-ala-trp-glu) + (val-ile) + (ile) | |
| (ala) + (ala) + (thr-val-ala-thr-asn-ala) | |
| (mal) + (pk-tyr-ala) + (ala) + (ser) + (ser-ile-ser) | |
| (asn) + (ala) + (ala) + (ala-gly) + (mal) + (ala-ala) + (val) | |
| (pk-ala) + (thr-ser-orn-ala-ala) + (phe-ala-ala) | |
| (leu-thr-dab-dab) + (thr) + (dab-thr-dab-dab-dab) | |
| (pk-gly) + (pk) + (mal) |
| Step/Fraction | Sample Weight (mg) | Total Activity (AU/mg) | Specific Activity (AU/mg) |
|---|---|---|---|
| Media Optimization | |||
| M 178 | 104.8 | 0 | 0 |
| TSB | 110.4 | 74.8 | 0.68 |
| TSB-S20 | 183 | 172.7 | 0.94 |
| TSB-S40 | 196.4 | 285.7 | 1.4 |
| LB | 175.2 | 35.3 | 0.20 |
| kYPD | 127.2 | 22.0 | 0.17 |
| Method of extraction | |||
| Acid precipitation | 71.3 | 0 | 0 |
| Amberlite XAD-7HP | 573 | 502.4 | 0.88 |
| MCI gel-HP | 418.5 | 50.2 | 0.12 |
| Diaion HP-20 | 162.8 | 22.0 | 0.13 |
| Chromatographic purification | |||
| HPLC timed fractionation | 35.4 | 335.1 | 9.5 |
| HPLC single-peak isolation | 23.54 | 362.8 | 15.4 |
| Strain | MIC (mg/mL) | Interpretation |
|---|---|---|
| P. aeruginosa MRSN 17849 | ≥4 | Resistant |
| P. aeruginosa MRSN 18560 | ≥4 | Resistant |
| P. aeruginosa MRSN 2108 | ≥4 | Resistant |
| A. baumannii AB-10 | ≤2 | Intermediate |
| K. pneumoniae KP-UTI-2 | ≤2 | Susceptible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chunduru, J.; LaRoe, N.; Garza, J.; Hamood, A.N.; Paré, P.W. Nosocomial Bacteria Inhibition with Polymyxin B: In Silico Gene Mining and In Vitro Analysis. Antibiotics 2024, 13, 745. https://doi.org/10.3390/antibiotics13080745
Chunduru J, LaRoe N, Garza J, Hamood AN, Paré PW. Nosocomial Bacteria Inhibition with Polymyxin B: In Silico Gene Mining and In Vitro Analysis. Antibiotics. 2024; 13(8):745. https://doi.org/10.3390/antibiotics13080745
Chicago/Turabian StyleChunduru, Jayendra, Nicholas LaRoe, Jeremy Garza, Abdul N. Hamood, and Paul W. Paré. 2024. "Nosocomial Bacteria Inhibition with Polymyxin B: In Silico Gene Mining and In Vitro Analysis" Antibiotics 13, no. 8: 745. https://doi.org/10.3390/antibiotics13080745