Pentasa Prescribing Information
Package insert / product label
Generic name: mesalamine
Dosage form: capsule
Drug class: 5-aminosalicylates
Medically reviewed by Drugs.com. Last updated on Aug 14, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
PENTASA® (mesalamine) extended-release capsules, for oral use
Initial U.S. Approval: 1987
Indications and Usage for Pentasa
PENTASA is an aminosalicylate indicated for the induction of remission and for the treatment of mildly to moderately active ulcerative colitis in adult patients. (1)
Pentasa Dosage and Administration
- Evaluate renal function prior to initiation of PENTASA and periodically while on therapy. (2, 5.1)
- The recommended dosage is 1 g administered orally four times daily. (2)
- Swallow capsules whole; do not crush or chew. (2)
- Alternatively, the capsule(s) may be opened and the contents sprinkled onto applesauce or yogurt. (2)
- Drink an adequate amount of fluids. (2, 5.7)
Dosage Forms and Strengths
Extended-release capsules: 250 mg and 500 mg. (3)
Contraindications
Warnings and Precautions
- Renal Impairment: Assess renal function at the beginning of treatment and periodically during treatment. Evaluate the risks and benefits of PENTASA in patients with known renal impairment or taking nephrotoxic drug. Discontinue PENTASA if renal function deteriorates while on therapy. (5.1, 7.1, 8.6)
- Mesalamine-Induced Acute Intolerance Syndrome: Discontinue treatment if acute intolerance syndrome (cramping, acute abdominal pain, bloody diarrhea, sometimes fever, headache and rash) is suspected. (5.2)
- Hypersensitivity Reactions, including myocarditis and pericarditis: Discontinue PENTASA if a hypersensitivity reaction is suspected. (5.3)
- Hepatic Failure: Evaluate the risks and benefits of using PENTASA in patients with known liver impairment. (5.4)
- Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.5)
- Photosensitivity: Avoid sun exposure if pre-existing skin conditions. (5.6)
- Nephrolithiasis: Cases of nephrolithiasis have been reported with the use of mesalamine. Mesalamine-containing stones are undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment. (5.7)
- Interference with Laboratory Tests: Mesalamine may lead to elevated urinary normetanephrine test results. (5.8)
Adverse Reactions/Side Effects
Most common adverse reactions are nausea and vomiting (1%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Nephrotoxic Agents including Non-Steroidal Anti-inflammatory Drugs (NSAIDs): Increased risk of nephrotoxicity; monitor for changes in renal function and mesalamine-related adverse reactions. (7.1)
- Azathioprine or 6-Mercaptopurine: Increased risk of blood dyscrasias; monitor complete blood cell counts and platelet counts. (7.2)
Use In Specific Populations
Geriatric Patients: Increased risk of blood dyscrasias; monitor complete blood cell counts and platelet counts. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2024
Full Prescribing Information
1. Indications and Usage for Pentasa
PENTASA is indicated for the induction of remission and for the treatment of mildly to moderately active ulcerative colitis in adult patients.
2. Pentasa Dosage and Administration
Evaluate renal function prior to initiation of PENTASA and periodically while on therapy [see Warnings and Precautions (5.1)].
Recommended Dosage
The recommended dosage for the induction of remission and the symptomatic treatment of mildly to moderately active ulcerative colitis in adults is 1 g (4 PENTASA 250 mg capsules or 2 PENTASA 500 mg capsules) administered orally four times daily.
Administration Instructions
- Swallow PENTASA capsules whole; do not crush or chew.
- Alternatively, the capsule(s) may be opened and the entire contents sprinkled onto applesauce or yogurt. Consume the entire mixture immediately.
- Drink an adequate amount of fluids during treatment [see Warnings and Precautions (5.7)].
3. Dosage Forms and Strengths
Extended-release capsules
- 250 mg as a green and blue capsule imprinted with a pentagonal starburst logo and the number 2010 on the green portion of the capsule and S429 250 mg on the blue portion of the capsule.
- 500 mg as a blue capsule imprinted with a pentagonal starburst logo and S429 500 mg.
4. Contraindications
PENTASA is contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates, or any ingredients of PENTASA [see Warnings and Precautions (5.3)].
5. Warnings and Precautions
5.1 Renal Impairment
Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and renal failure have been reported in patients given PENTASA or other products that contain mesalamine or are converted to mesalamine.
Evaluate the risks and benefits of using PENTASA in patients with known renal impairment or a history of renal disease or taking concomitant nephrotoxic drugs. Evaluate renal function in all patients prior to initiation and periodically while on therapy with PENTASA. Discontinue PENTASA if renal function deteriorates while on therapy [see Drug Interactions (7.1), Use in Specific Populations (8.6)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Symptoms include cramping, acute abdominal pain, bloody diarrhea, and sometimes fever, headache, and rash. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with PENTASA.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions have been reported in patients taking sulfasalazine. Some patients may have a similar reaction to PENTASA or to other compounds that contain or are converted to mesalamine.
As with sulfasalazine, mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis, and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue PENTASA if an alternative etiology for the signs and symptoms cannot be established.
5.4 Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered other products containing mesalamine. Evaluate the risks and benefits of using PENTASA in patients with known liver impairment.
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported in with the use of mesalamine [see Adverse Reactions (6.2)]. Discontinue PENTASA at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Photosensitivity
Patients with pre-existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
5.7 Nephrolithiasis
Cases of nephrolithiasis have been reported with the use of mesalamine, including stones with 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment with PENTASA.
5.8 Interference with Laboratory Tests
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and mesalamine’s main metabolite, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). Consider an alternative, selective assay for normetanephrine.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Renal impairment [see Warnings and Precautions (5.1)]
- Mesalamine-induced acute intolerance syndrome [see Warnings and Precautions (5.2)]
- Hypersensitivity reactions [see Warnings and Precautions (5.3)]
- Hepatic failure [see Warnings and Precautions (5.4)]
- Severe cutaneous adverse reactions [see Warnings and Precautions (5.5)]
- Photosensitivity [see Warnings and Precautions (5.6)]
- Nephrolithiasis [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
More than 2100 patients were exposed to PENTASA in clinical trials of ulcerative colitis or another gastrointestinal condition. The most common adverse reactions (i.e., greater than or equal to 1%) were diarrhea (3%), headache (2%), nausea (2%), abdominal pain (2%), dyspepsia (2%), vomiting (2%), and rash (1%).
The safety of PENTASA was evaluated in two randomized, double-blind, placebo-controlled, dose-response trials (UC-1 and UC-2) of 624 patients with mildly to moderately active ulcerative colitis for up to 8 weeks of treatment [see Clinical Studies (14)]. The most common adverse reaction was nausea and vomiting: 1% in the PENTASA group (N=451) and 0% in the placebo group (N=173). Withdrawal from therapy due to adverse reactions was 7% in the PENTASA group and 4% in the placebo group.
The following adverse reactions, presented by body system, were reported in less than 1% of patients in UC-1, UC-2, and clinical trials for another gastrointestinal condition.
Blood and lymphatic system disorders: thrombocythemia, thrombocytopenia
Cardiac Disorders: palpitations, pericarditis, vasodilation
Gastrointestinal Disorders: abdominal distention, constipation, duodenal ulcer, dysphagia, eructation, esophageal ulcer, fecal incontinence, GI bleeding, mouth ulcer, pancreatitis, rectal bleeding, stool abnormalities (color or texture change)
General disorders and administration site conditions: fever, malaise
Infections and infestations: oral moniliasis, conjunctivitis
Investigations: GGTP increase, increased alkaline phosphatase, LDH increase, SGOT increase, SGPT increase, lipase increase, amylase increase
Metabolism and nutritional disorders: anorexia, edema, thirst
Musculoskeletal and connective tissue disorders: arthralgia, leg cramps, myalgia
Nervous System Disorders: dizziness, insomnia, somnolence, paresthesia
Psychiatric disorders: depression, asthenia
Renal and urinary disorders: albuminuria, hematuria, urinary frequency
Reproductive system and breast disorders: amenorrhea, breast pain, hypomenorrhea, menorrhagia, metrorrhagia
Respiratory, Thoracic and Mediastinal Disorders: pulmonary infiltrates, one week after completion of an 8-week ulcerative colitis study, a 72-year-old male, with no previous history of pulmonary problems, developed dyspnea. The patient was subsequently diagnosed with interstitial pulmonary fibrosis without eosinophilia by one physician and bronchiolitis obliterans with organizing pneumonitis by a second physician.
Skin and Subcutaneous Tissue Disorders: acne, alopecia, dry skin, eczema, erythema nodosum, nail disorder, photosensitivity, pruritus, sweating, urticaria, ecchymosis, lichen planus
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of mesalamine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders: chest pain, fatal myocarditis, pericarditis, T-wave abnormalities
Hematologic Disorders: agranulocytosis, anemia, aplastic anemia, leukopenia, pancytopenia
Hepatic Disorders: cirrhosis, jaundice, including cholestatic jaundice; hepatotoxicity, hepatitis, and possible hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal. One case of Kawasaki-like syndrome which included hepatic function changes was also reported.
Immune System Disorders: anaphylactic reaction, angioedema, lupus-like syndrome, systemic lupus erythematosus
Nervous System Disorders: intracranial hypertension
Renal and Urinary Disorders: acute renal failure, chronic renal failure, interstitial nephritis, nephrogenic diabetes insipidus, nephrolithiasis, nephrotic syndrome [see Warnings and Precautions (5.1, 5.7)]
- Urine discoloration occurring ex-vivo caused by contact of mesalamine, including inactive metabolite, with surfaces or water treated with hypochlorite-containing bleach
Reproductive System and Breast Disorders: reversible oligospermia
Respiratory, Thoracic and Mediastinal Disorders: hypersensitivity pneumonitis (including interstitial pneumonitis, allergic alveolitis, eosinophilic pneumonitis), interstitial lung disease, pleurisy/pleuritis, pneumonitis
Skin and Subcutaneous Tissue Disorders: AGEP, DRESS, SJS/TEN [see Warnings and Precautions (5.5)]
7. Drug Interactions
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs), may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions [see Warnings and Precautions (5.1)].
7.2 Azathioprine or 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine and/or any other drugs known to cause myelotoxicity may increase the risk for blood disorders, bone marrow failure, and associated complications. If concomitant use of PENTASA and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
7.3 Interference with Urinary Normetanephrine Measurements
Use of PENTASA may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection [see Warnings and Precautions (5.8)]. Consider an alternative, selective assay for normetanephrine.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Published data from meta-analyses, cohort studies, and case series on the use of mesalamine during pregnancy have not reliably informed an association with mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are adverse effects on maternal and fetal outcomes associated with ulcerative colitis in pregnancy (see Clinical Considerations).
In animal reproduction studies, oral administration of mesalamine during organogenesis to pregnant rats at doses up to 1000 mg/kg/day (approximately 2.4 times the maximum recommended human dose of 4 g/day, based on a body surface area comparison) and rabbits at doses of 800 mg/kg/day (approximately 3.9 times the maximum recommended human dose of 4 g/day, based on a body surface area comparison) revealed no evidence of adverse developmental effects (see Data).
The background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with ulcerative colitis. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
Data
Human Data
Published data from meta-analyses, cohort studies, and case series on the use of mesalamine during early pregnancy (first trimester) and throughout pregnancy have not reliably informed an association of mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes. There is no clear evidence that mesalamine exposure in early pregnancy is associated with an increased risk of major congenital malformations, including cardiac malformations. Published epidemiologic studies have important methodological limitations which hinder interpretation of the data, including inability to control for confounders, such as underlying maternal disease, maternal use of concomitant medications, and missing information on the dose and duration of use for mesalamine products.
Animal Data
Reproduction studies with mesalamine during organogenesis have been performed in pregnant rats at doses up to 1000 mg/kg/day (approximately 2.4 times the maximum recommended human dose of 4 g/day, based on a body surface area comparison) and rabbits at doses up to 800 mg/kg/day (approximately 3.9 times the maximum recommended human dose of 4 g/day based on a body surface area comparison) and have revealed no evidence of harm to the fetus due to mesalamine.
8.2 Lactation
Risk Summary
Data from published literature report the presence of mesalamine and its metabolite, N-acetyl-5-aminosalicylic acid in human milk in small amounts with relative infant doses (RID) of 0.1% or less for mesalamine (see Data). There are case reports of diarrhea observed in breastfed infants exposed to mesalamine (see Clinical Considerations). There is no information on the effects of mesalamine on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of PENTASA to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PENTASA and any potential adverse effects on the breastfed child from PENTASA or from the underlying maternal condition.
Data
In published lactation studies, maternal mesalamine doses from various oral and rectal formulations and products ranged from 500 mg to 4.8 g daily. The average concentration of mesalamine in milk ranged from non-detectable to 0.5 mg/L. The average concentration of N-acetyl-5-aminosalicylic acid in milk ranged from 0.2 to 9.3 mg/L. Based on these concentrations, estimated infant daily dosages for an exclusively breastfed infant are 0 to 0.075 mg/kg/day of mesalamine (RID 0% to 0.1%) and 0.03 to 1.4 mg/kg/day of N-acetyl-5-aminosalicylic acid.
8.5 Geriatric Use
Clinical trials of PENTASA did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias (i.e., agranulocytosis, neutropenia, and pancytopenia) in patients receiving mesalamine-containing products such as PENTASA who were 65 years or older compared to younger adult patients, which may also be associated with ulcerative colitis, use of interacting drugs, or reduced renal function. Monitor complete blood cell counts and platelet counts in patients 65 years and over during treatment with PENTASA.
In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concurrent disease or other drug therapy in patients 65 years and over when prescribing PENTASA.
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on PENTASA therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions. Discontinue PENTASA if renal function deteriorates while on therapy [see Warnings and Precautions (5.1), Adverse Reactions (6.2), Drug Interactions (7.1)].
10. Overdosage
PENTASA is an aminosalicylate, and symptoms of salicylate toxicity may be possible, such as: nausea, vomiting, abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe intoxication with salicylates may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) damage.
There is no specific antidote for mesalamine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage and may include gastrointestinal tract decontamination to prevent further absorption. Correct fluid and electrolyte imbalance by the administration of appropriate intravenous therapy and maintain adequate renal function.
11. Pentasa Description
PENTASA (mesalamine) for oral administration is an extended-release formulation of mesalamine, an aminosalicylate anti-inflammatory agent for gastrointestinal use.
Mesalamine (also referred to as 5-aminosalicylic acid or 5-ASA) has the chemical name 5-amino-2-hydroxybenzoic acid. It has a molecular weight of 153.14.
The structural formula is:
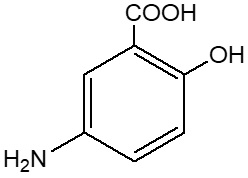 |
Each 250 mg capsule contains 250 mg of mesalamine. It also contains the following inactive ingredients: acetylated monoglyceride, castor oil, colloidal silicon dioxide, ethylcellulose, hydroxypropyl methylcellulose, starch, stearic acid, sugar, talc, and white wax. The capsule shell contains D&C Yellow #10, FD&C Blue #1, FD&C Green #3, gelatin, titanium dioxide, and other ingredients.
Each 500 mg capsule contains 500 mg of mesalamine. It also contains the following inactive ingredients: acetylated monoglyceride, castor oil, colloidal silicon dioxide, ethylcellulose, hydroxypropyl methylcellulose, starch, stearic acid, sugar, talc, and white wax. The capsule shell contains FD&C Blue #1, gelatin, titanium dioxide, and other ingredients.
12. Pentasa - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of mesalamine is not fully understood, but it appears to be a topical anti-inflammatory effect on colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways (i.e., prostanoids) and through the lipoxygenase pathways (i.e., leukotrienes and hydroxyeicosatetraenoic acids), is increased in patients with ulcerative colitis, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of mesalamine have not been fully characterized.
12.3 Pharmacokinetics
Absorption
Following oral administration 20% to 30% of the mesalamine in PENTASA is absorbed based on urinary excretion data.
Plasma mesalamine concentration peaked at approximately 1 mcg/mL 3 hours following a 1 g PENTASA dose.
Oral mesalamine pharmacokinetics were nonlinear when PENTASA capsules were dosed from 250 mg to 1 g four times daily, with steady-state mesalamine plasma concentrations increasing about nine times, from 0.14 mcg/mL to 1.21 mcg/mL.
The major metabolite of mesalamine (5-aminosalicylic acid), N-acetyl-5-aminosalicylic acid, peaked at approximately 3 hours at 1.8 mcg/mL. N-acetyl-5-aminosalicylic acid pharmacokinetics were linear.
Distribution
Mesalamine is approximately 43% bound to plasma proteins at the concentration of 2.5 mcg/mL.
Elimination
Metabolism
The greater than dose proportional increase in PK of mesalamine suggests saturable first pass metabolism. The major metabolite of mesalamine, N-acetyl-5-aminosalicylic acid, is formed due to action of N-acetyltransferase in the liver and intestinal mucosa. Pharmacological activities of N-acetyl-5-aminosalicylic acid are unknown, and other metabolites have not been identified.
Excretion
Following oral administration, plasma mesalamine concentration declined in a biphasic manner after reaching peak concentration. The literature describes a mean terminal half-life of 42 minutes for mesalamine following intravenous administration. Because of the continuous release and absorption of mesalamine from PENTASA throughout the gastrointestinal tract, the true elimination half-life cannot be determined after oral administration.
N-acetyl-5-aminosalicylic acid was the primary compound excreted in the urine (19% to 30%) following PENTASA dosing. About 130 mg free mesalamine was recovered in the feces following a single 1g PENTASA dose. Elimination of free mesalamine and salicylates in feces increased proportionately with PENTASA dose.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 104-week dietary carcinogenicity study of mesalamine, CD-1 mice were administered doses up to 2500 mg/kg/day and it was not tumorigenic. The 2500 mg/kg/day dose represents approximately 2.5 times the maximum recommended human dose on a body surface area basis. In a 104-week dietary carcinogenicity study in Wistar rats, mesalamine up to a dose of 800 mg/kg/day was not tumorigenic. This dose represents approximately 1.5 times the maximum recommended human dose on a body surface area basis.
Mutagenesis
No evidence of mutagenicity was observed in an in vitro Ames test and in an in vivo mouse micronucleus test.
Impairment of Fertility
No effects on fertility or reproductive performance were observed in male or female rats at oral doses of mesalamine up to 400 mg/kg/day (0.8 times the maximum recommended human dose based on body surface area).
14. Clinical Studies
In two randomized, double-blind, placebo-controlled, dose-response trials (UC-1 and UC-2) of 625 patients with active mild to moderate ulcerative colitis, PENTASA, at an oral dose of 1 g administered four times a day for up to 8 weeks, produced consistent improvement in prospectively identified primary efficacy parameters, Physical Global Assessment, Treatment Failure, and Sigmoidoscopic Index as shown in Table 2.
A PENTASA dosage regimen of 1 g four times a day demonstrated consistent improvement in secondary efficacy parameters, namely the frequency of trips to the toilet, stool consistency, rectal bleeding, abdominal/rectal pain, and urgency. A PENTASA dosage regimen of 1 g four times a day also induced remission as assessed by endoscopic and symptomatic endpoints.
| 1 proportion of patients with complete or marked improvement. | ||||
| 2 proportion of patients developing severe or fulminant UC requiring steroid therapy or hospitalization or worsening of the disease at 7 days of therapy, or lack of significant improvement by 14 days of therapy. | ||||
| 3 an objective measure of disease activity rated by a standard (15-point) scale that includes mucosal vascular pattern, erythema, friability, granularity/ulcerations, and mucopus: improvement over baseline. | ||||
| 4 defined as complete resolution of symptoms plus improvement of endoscopic endpoints. To be considered in remission, patients had a “1” score for one of the endoscopic components (mucosal vascular pattern, erythema, granularity, or friability) and “0” for the others. | ||||
|
||||
| Parameter Evaluated | Clinical Trial UC-1 | Clinical Trial UC-2 | ||
| Placebo N=90 | PENTASA 1 g four times a day N=95 | Placebo N=83 | PENTASA 1 g four times a day N=85 |
|
| Physician Global Assessment1 | 36% | 59%* | 31% | 55%* |
| Treatment Failure2 | 22% | 9%* | 31% | 9%* |
| Sigmoidoscopic Index3 | -2.5 | -5.0* | -1.6 | -3.8* |
| Remission4 | 12% | 26%* | 12% | 27%* |
16. How is Pentasa supplied
PENTASA (mesalamine) extended-release capsules are supplied as shown in the table:
| Strength | Description | Supplied As | NDC Number |
|---|---|---|---|
| 250 mg extended-release capsules | green and blue capsule with a pentagonal starburst logo and the number 2010 on the green portion of the capsule and S429 250 mg on the blue portion of the capsule | bottles of 240 capsules | NDC 54092-189-81 |
| 500 mg extended-release capsules | blue capsule with a pentagonal starburst logo and S429 500 mg on the capsule | bottles of 120 capsules | NDC 54092-191-12 |
17. Patient Counseling Information
Renal Impairment
Inform patients that PENTASA may decrease their renal function, especially if they have known renal impairment or are taking nephrotoxic drugs, and periodic monitoring of renal function will be performed while they are on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Warnings and Precautions (5.1)].
Mesalamine-Induced Acute Intolerance Syndrome and Other Hypersensitivity Reactions
Instruct patients to stop taking PENTASA and report to their healthcare provider if they experience new or worsening symptoms of acute intolerance syndrome (cramping, abdominal pain, bloody diarrhea, fever, headache, and rash) or other symptoms suggestive of mesalamine-induced hypersensitivity [see Warnings and Precautions (5.2, 5.3)].
Hepatic Failure
Advise patients with known liver disease to contact their healthcare provider if they experience signs or symptoms of worsening liver function [see Warnings and Precautions (5.4)].
Severe Cutaneous Adverse Reactions
Inform patients of the signs and symptoms of severe cutaneous adverse reactions. Instruct patients to stop taking PENTASA and report to their healthcare provider at first appearance of a severe cutaneous adverse reaction or other sign of hypersensitivity [see Warnings and Precautions (5.5)].
Photosensitivity
Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors [see Warnings and Precautions (5.6)].
Nephrolithiasis
Instruct patients to drink an adequate amount of fluids during treatment in order to minimize the risk of kidney stone formation and to contact their healthcare provider if they experience signs or symptoms of a kidney stone (e.g., severe side or back pain, blood in the urine) [see Warnings and Precautions (5.7)].
Blood Disorders
Inform elderly patients and those taking azathioprine or 6-mercaptopurine of the risk for blood disorders and the need for periodic monitoring of complete blood cell counts and platelet counts while on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Drug Interactions (7.2), Use in Specific Populations (8.5)].
Administration
Instruct patients:
- Swallow PENTASA capsules whole; do not crush or chew.
- Alternatively, the capsule(s) may be opened and the contents sprinkled onto applesauce or yogurt.
- Urine may become discolored reddish-brown while taking PENTASA when it comes in contact with surfaces or water treated with hypochlorite-containing bleach. If discolored urine is observed, advise patients to observe their urine flow. Report to the healthcare provider only if urine is discolored on leaving the body, before contact with any surface or water (e.g., in the toilet).
- Drink an adequate amount of fluids [see Dosage and Administration (2), Warnings and Precautions (5.7)].
Distributed by:
Takeda Pharmaceuticals America, Inc.
Cambridge, MA 02142, USA
PENTASA® is a registered trademark of Ferring B.V. used under license.
© 2024 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
Rev. 7/2024
| PENTASA
mesalamine capsule |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| PENTASA
mesalamine capsule |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Manufacturing Services LLC | 079415560 | ANALYSIS(54092-189, 54092-191) , LABEL(54092-189, 54092-191) , MANUFACTURE(54092-189, 54092-191) , PACK(54092-189, 54092-191) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PharmaZell GmbH | 506639652 | API MANUFACTURE(54092-189, 54092-191) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Syntese A/S | 305104267 | ANALYSIS(54092-189, 54092-191) , API MANUFACTURE(54092-189, 54092-191) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Packaging Services, LLC | 143696495 | PACK(54092-189, 54092-191) | |
Frequently asked questions
More about Pentasa (mesalamine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (89)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Drug class: 5-aminosalicylates
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Lialda, Apriso, Asacol, Canasa, ... +4 more

