More about...Paediatric cardiology
The role of epigenetics in the
origin of congenital heart
disease
Rik De Decker, MSc, MB ChB, DCH,
FCPaeds (SA), Cert Med Genetics
(Paeds)
Senior specialist paediatric cardiologist,
Division of Critical Care and Children’s Heart
Diseases, Red Cross War Memorial Children’s
Hospital, Cape Town
Correspondence to: Rik De Decker (rik.dedecker@
uct.ac.za)
The past decade has seen a remarkable
explosion of insights into the control of
heart development. This has come about as
a consequence of the temporal confluence
of several research domains: the better
delineation of syndromic congenital
heart disease (see article in this issue),
the completion of the Human Genome
Project in 2003, the remarkable advances
in molecular embryology and a deeper
understanding of genetic evolution. It is
against this background, and by combining
data from anatomical embryology, early fetal
myocardial function, and fetal blood flow
with a new understanding of the genetically
modular development of cardiac chambers
(the independent, regional development
of cardiac chambers under distinct genetic
control) that the ballooning model of
heart development1 has revolutionised our
understanding of normal heart development.
The evolution of endothermic physiology
required the efficiency of a four-chambered
heart, with two parallel but separate
circulations: systemic and pulmonary. The
complexity of its development from a single
heart tube in the very early fetus allows for
little redundancy, and relatively small lesions
may compromise metabolic efficiency
severely, leading to heart disease and early
death. It seems reasonable to assume that
most, if not all, congenital heart disease
(CHD) stems from errors in the genetic
control of heart development, and that the
understanding of these controlling molecular
mechanisms may allow us to understand the
origins of CHD. And with understanding
comes the potential of prevention and
possibly even early repair.
Some questions arise, however
• If all cells have essentially exactly the
same DNA, how is differentiation during
development controlled? A nerve cell
is fundamentally different from a right
ventricular myocyte, which differs
markedly from a cell in the sino-atrial
node!
• The birth incidence of CHD is
approximately 8/1 000, of which 80% is
sporadic (non-syndromic): if sporadic
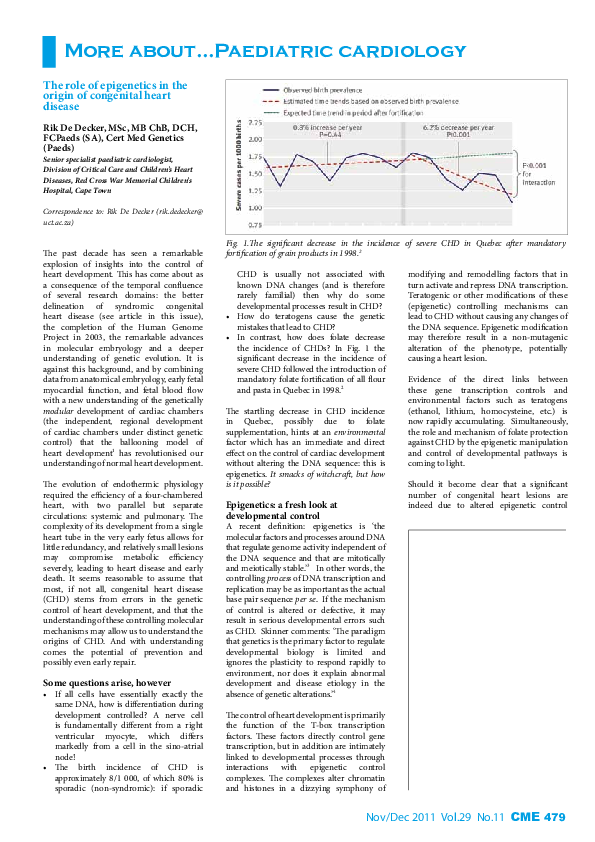
Fig. 1.The significant decrease in the incidence of severe CHD in Quebec after mandatory
fortification of grain products in 1998.2
CHD is usually not associated with
known DNA changes (and is therefore
rarely familial) then why do some
developmental processes result in CHD?
• How do teratogens cause the genetic
mistakes that lead to CHD?
• In contrast, how does folate decrease
the incidence of CHDs? In Fig. 1 the
significant decrease in the incidence of
severe CHD followed the introduction of
mandatory folate fortification of all flour
and pasta in Quebec in 1998.2
The startling decrease in CHD incidence
in Quebec, possibly due to folate
supplementation, hints at an environmental
factor which has an immediate and direct
effect on the control of cardiac development
without altering the DNA sequence: this is
epigenetics. It smacks of witchcraft, but how
is it possible?
Epigenetics: a fresh look at
developmental control
modifying and remodelling factors that in
turn activate and repress DNA transcription.
Teratogenic or other modifications of these
(epigenetic) controlling mechanisms can
lead to CHD without causing any changes of
the DNA sequence. Epigenetic modification
may therefore result in a non-mutagenic
alteration of the phenotype, potentially
causing a heart lesion.
Evidence of the direct links between
these gene transcription controls and
environmental factors such as teratogens
(ethanol, lithium, homocysteine, etc.) is
now rapidly accumulating. Simultaneously,
the role and mechanism of folate protection
against CHD by the epigenetic manipulation
and control of developmental pathways is
coming to light.
Should it become clear that a significant
number of congenital heart lesions are
indeed due to altered epigenetic control
A recent definition: epigenetics is ‘the
molecular factors and processes around DNA
that regulate genome activity independent of
the DNA sequence and that are mitotically
and meiotically stable.’3 In other words, the
controlling process of DNA transcription and
replication may be as important as the actual
base pair sequence per se. If the mechanism
of control is altered or defective, it may
result in serious developmental errors such
as CHD. Skinner comments: ‘The paradigm
that genetics is the primary factor to regulate
developmental biology is limited and
ignores the plasticity to respond rapidly to
environment, nor does it explain abnormal
development and disease etiology in the
absence of genetic alterations.’4
The control of heart development is primarily
the function of the T-box transcription
factors. These factors directly control gene
transcription, but in addition are intimately
linked to developmental processes through
interactions with epigenetic control
complexes. The complexes alter chromatin
and histones in a dizzying symphony of
Nov/Dec 2011 Vol.29 No.11 CME 479
�More about
of DNA transcription, thereby leading to
maldevelopment, the potential exists that
these mechanisms are targets of future
preventive or therapeutic interventions.
DNA, we thought, was an iron-clad
code that we and our children and
their children had to live by. Now we
can imagine a world in which we tinker
with DNA, bend it to our will. It will
take geneticists and ethicists many years
to work out all the implications, but
be assured: the age of epigenetics has
arrived.
is not generating a co-ordinated, perfusing
rhythm. Organised QRS complexes cannot
be identified and the electrical current is
delivered without synchronising with the
patient’s native rhythm. DC shock should
not be delayed once a shockable rhythm is
recognised. The longer the time delay the
worse the outcome. CPR should continue
while preparing the defibrillator. Care
should be taken to clear all involved, and the
oxygen should be cleared before discharging
the current. CPR should resume (starting
with compressions) immediately after the
DC shock and continued for five cycles (2
minutes) before the next rhythm check.
John Cloud, Time, January 2010.
References and further reading available at
www.cmej.org.za
Defibrillation and
cardioversion in children:
demystifying the shock of
shocking
Beyra Rossouw, MB ChB, MMed
(Paed), DTM, MSc (Sports Medicine),
Certificate Critical Care (Paed)
Senior Registrar Paediatric Cardiology, Western
Cape Paediatric Cardiac Services, Red Cross
War Memorial Children’s Hospital, University of
Cape Town, and Tygerberg Children’s Hospital,
Stellenbosch University
Correspondence to: B Rossouw (beyra@sun.ac.za)
Health care practitioners looking after
children are often uncomfortable about
using direct current (DC) shock treatment
on a child. This article emphasises practical
points when using electrical shock therapy
in children, but does not replace the value of
attending an APLS course to gain hands-on
experience.
The most common life-threatening
dysrhythmias in children are non-shockable
rhythms, mostly due to hypoxia. However,
childhood shockable dysrhythmias cannot
be considered as rare. These include
ventricular fibrillation (VF), pulseless
ventricular
tachycardia
(VT)
and
supraventricular tachycardia (SVT).
Recent reports indicate that as many as 25%
of in-hospital cardiac arrests in children
and 5 - 22% of out-of-hospital paediatric
cardiac arrests are due to VF or pulseless VT.
Shockable dysrhythmias are more likely to
present in children with an underlying cardiac
disease, or present as a sudden collapse.
Defibrillation
Defibrillation indicates a DC shock treatment
aimed at depolarising a myocardium that
Defibrillation energy dose
The optimal and safe defibrillation energy
dose in children is unknown. The risk of
myocardial damage when using higher
electrical currents should be considered
against using lower energy but wasting
time before achieving a stable rhythm.
The International Liaison Committee on
Resuscitation recommends an initial dose of
2 J/kg, thereafter 4 J/kg. Evidence suggests
that more than 4 J/kg (biphasic defibrillator)
is effective and safe. Some defibrillators
provide limited manual joule options. When
dialling in the weight-based energy on the
defibrillator, round the number down to the
lower joule setting.
Modern defibrillators deliver biphasic
shocks as opposed to monophasic shocks.
Biphasic shocks are more effective and
cause less myocardial damage. Biphasic
currents are delivered in two phases: first a
positive current in one direction and then a
negative current from the opposite direction.
Evidence in adults suggests a survival benefit
in single shock versus stacked shocks.
Transthoracic impedance is the primary
determinant of effective energy delivery.
Measures to reduce the transthoracic
impedance include: firm contact between the
paddle and the chest, larger paddle size and
electrolyte-containing gel.
Paddles and positions
Paediatric-sized paddles should be used in
children under 1 year of age (<10 kg) and
adult-sized paddles in those older than 1
year (>10 kg). One paddle should be below
the right clavicle parallel to the sternum and
the other parallel to the first paddle in the
left axilla to optimise the energy transfer.
Paddles should be applied firmly, parallel to
each other, with at least a 3 kg force applied
onto paddles for infants and a 5 kg force for
children.
Defibrillation gel reduces the transthoracic
impedance. KY jelly, sonar gel, alcohol- or
saline-soaked gauze should not be used
as alternatives. Take care that the gel does
480 CME Nov/Dec 2011 Vol.29 No.11
not smear over the chest wall and cause
potential arcing (i.e. the current flows over
the chest between the paddles and not into
the chest). DC shock should ideally be
discharged on end-expiration to minimise
impedance.
Larger paddles reduce impedance but risk
arcing of the current if the paddles are too
close. There should be at least 3 cm between
the paddles. In the case of a small chest and
large paddles, use the anterior-posterior
paddle position to prevent arcing: one
paddle is placed below the left scapula and
the other parallel to the left of the sternum.
It does not matter which paddle is placed in
which position.
Cardioversion
The terms defibrillation and cardioversion
are often wrongly used interchangeably.
Cardioversion is applied to a myocardium
with an abnormal rhythm that is able
to generate a pulse, but insufficient for
adequate perfusion. Defibrillation is used
when there is no pulse or no perfusing
rhythm. Cardioversion is used for patients
with haemodynamic unstable SVT, VT
(with a pulse), atrial fibrillation and atrial
flutter.
The energy dose in cardioversion is less (0.5
- 2 J/kg) than in defibrillation (2 - 4 J/kg).
In cardioversion the shock is discharged
synchronously with the native R wave
of the patient. Without synchronisation,
VF can be induced if a shock is delivered
during the refractory period of the cardiac
cycle. The majority of defibrillators default
to unsynchronised mode. It is therefore
imperative to reset the synchronisation button
before each discharge. Synchronisation with
a broad complex VT can be difficult. Choose
the lead with the best identifiable R waves.
Synchronisation problems must be suspected
when the defibrillator fails to discharge after
pressing the shock button. In this case use
unsynchronised cardioversion.
Children with congenital heart disease
are now surviving into adulthood.
Unfortunately cardiac surgery leaves atrial
scars that may predispose the patient to
dysrhythmias. Therefore life-threatening
shockable dysrhythmias will be seen more
often in the emergency setting. Healthcare
practitioners should aim to deliver the first
DC shock within 3 minutes after recognising
the shockable arrhythmia.
Suggested reading available at www.cmej.org .za
�
 Rik De Decker
Rik De Decker