Evaluation of different culture media to support in vitro growth and biofilm formation of bacterial vaginosis-associated anaerobes
- Published
- Accepted
- Received
- Academic Editor
- Joseph Gillespie
- Subject Areas
- Microbiology, Gynecology and Obstetrics, Women’s Health
- Keywords
- Bacterial vaginosis, BV-associated anaerobes, Anaerobic growth, Biofilms
- Copyright
- © 2020 Rosca et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Evaluation of different culture media to support in vitro growth and biofilm formation of bacterial vaginosis-associated anaerobes. PeerJ 8:e9917 https://doi.org/10.7717/peerj.9917
Abstract
Background
Bacterial vaginosis (BV) is one of the most common vaginal infections worldwide. It is associated with the presence of a dense polymicrobial biofilm on the vaginal epithelium, formed mainly by Gardnerella species. The biofilm also contains other anaerobic species, but little is known about their role in BV development.
Aim
To evaluate the influence of different culture media on the planktonic and biofilm growth of six cultivable anaerobes frequently associated with BV, namely Gardnerella sp., Atopobium vaginae, Lactobacillus iners, Mobiluncus curtisii, Peptostreptococcus anaerobius and Prevotella bivia.
Methods
A total of nine different culture media compositions, including commercially available and chemically defined media simulating genital tract secretions, were tested in this study. Planktonic cultures and biofilms were grown under anaerobic conditions (10% carbon dioxide, 10% helium and 80% nitrogen). Planktonic growth was assessed by optical density measurements, and biofilm formation was quantified by crystal violet staining.
Results
Significant planktonic growth was observed for Gardnerella sp., A. vaginae and L. iners in New York City III broth, with or without ascorbic acid supplementation. Biofilm quantification showed high in vitro biofilm growth for Gardnerella sp., P. anaerobius and P. bivia in almost all culture media excluding Brucella broth. Contrary, only New York City III broth was able to promote biofilm formation for A. vaginae, L. iners and M. curtisii.
Conclusions
Our data demonstrate that New York City III broth relative to the other tested media is the most conducive for future studies addressing polymicrobial biofilms development as this culture medium allowed the formation of significant levels of single-species biofilms.
Introduction
Bacterial vaginosis (BV) is the worldwide leading bacterial vaginal infection commonly recognized in women of all ethnicities between menarche and menopause (Beamer et al., 2017; Javed, Parvaiz & Manzoor, 2019). BV is often characterized by a decrease of beneficial vaginal bacteria, mainly hydrogen peroxide and lactic acid-producing Lactobacillus species, and by an increase of anaerobic pathogens (Marrazzo, 2011; Schwebke, 2000). The most prominent of these is Gardnerella vaginalis, a facultative anaerobe usually found embedded in a polymicrobial biofilm (Swidsinski et al., 2014). It is important to mention that an emended description of G. vaginalis was recently proposed with the delineation of 13 genomic species within the genus Gardnerella (Vaneechoutte et al., 2019). Of these 13 species, three were officially described (G. leopoldii, G. piotii, and G. swidsinskii) and G. vaginalis was amended. Following this renewed taxonomy of the genus Gardnerella, in this article, the term Gardnerella spp. will be used to address previous publications, since we cannot rule out the fact that other Gardnerella species were involved.
According to the current hypothesis for BV pathogenesis, Gardnerella spp. initiate the formation of the biofilm on vaginal epithelial cells and become a scaffolding to which other BV-associated species thereafter can attach (Muzny et al., 2019). One of the species that is often found associated with Gardnerella spp. biofilms is Atopobium vaginae (Swidsinski et al., 2005). Under specific in vitro conditions, G. vaginalis enhances culturability of A. vaginae (Castro et al., 2020) and it has been suggested that the in vivo detection of both bacteria is a strong indicator of BV development (Bradshaw et al., 2006; Hardy et al., 2016, 2015; Muzny et al., 2019). However, the BV biofilm is often populated by many other facultative or strict anaerobes, but very little is known about their role in BV development. As such, more studies are needed to address the interactions between these species. One issue facing researchers that work with BV-associated species is that most species are uncultivable or fastidious (Diop et al., 2019; Fredricks et al., 2007; Fredricks, Fiedler & Marrazzo, 2005; Srinivasan et al., 2016). Furthermore, in vitro biofilm formation requirements are often different from planktonic growth (Alves et al., 2014). Considering the increased focus on biofilm-associated infections and the demand for finding novel treatment approaches (Falconi-McCahill, 2019), the current study was undertaken aiming to evaluate the effects of nine different culture media on the planktonic growth and biofilm formation of cultivable anaerobes frequently found in BV, namely Gardnerella sp., A. vaginae, Mobiluncus curtisii, Peptostreptococcus anaerobius and Prevotella bivia (Diop et al., 2019; Onderdonk, Delaney & Fichorova, 2016). Importantly, Lactobacillus iners was also included in this study as this species plays a controversial role in the vaginal microenvironment, being detected in the vaginal microbiota of both healthy (Anukam et al., 2006; Ravel et al., 2011) and women with BV (Rampersaud et al., 2011). Furthermore, L. iners has been often identified in the intermediate vaginal microbiota (i.e., between normal and BV microbiota) (Jakobsson & Forsum, 2007; Petrova et al., 2015) and also dominates the microbiota after treatment of BV (Ferris et al., 2007). Also, this microorganism has complex nutritional requirements, being thus, not easy to work with in vitro (Vaneechoutte, 2017).
As such, the main goal of this study was to explore growth conditions optimal for using in future in vitro multi-species biofilm model, with the aim to better analyze BV multi-species interactions and their impact on BV development.
Materials and Methods
Bacterial species and growth conditions
Six cultivable bacterial species associated with BV were used in the current study, namely Gardnerella sp., A. vaginae, L. iners, M. curtisii, P. anaerobius and P. bivia (Table 1). These species were preserved frozen in Brain Heart Infusion broth (BHI) (Liofilchem, Italy) with 23% (v/v) glycerol (Panreac, Spain) at −80 °C. Each species was inoculated from the −80 °C bacterial stock on plates containing Columbia Blood Agar medium (CBA) (Oxoid, Basingstoke, UK) supplemented with 5% (v/v) defibrinated horse blood (Oxoid, Basingstoke, UK) and incubated at 37 °C under anaerobic conditions [controlled atmosphere composed of 10% carbon dioxide (CO2), 10% helium and 80% nitrogen generated by a cylinder (Air Liquid, Algés, Portugal) coupled to an anaerobic incubator (Plas-Labs, Lansing, MI, USA)] for 2–4 days. For planktonic and biofilm assays, Brain heart infusion broth supplemented with yeast extract, starch and gelatin (sBHI), Brucella broth supplemented with hemin and vitamin K1 solutions (BHV), New York City III broth supplemented with 10% (v/v) inactivated horse serum (NYC), Schaedler broth (SB), and a medium simulating genital tract secretions (mGTS) were used as culture media with the mentioned composition, but also supplemented with 0.1% (w/v) L-ascorbic acid (Sigma–Aldrich, Gillingham, UK), excepting mGTS which already contains L-ascorbic acid. The addition of L-ascorbic acid to the culture media was designated with the abbreviation “Aa”, added at the end of each medium’s name mentioned above (e.g., sBHI supplemented with L-ascorbic acid became sBHI.Aa). The detailed information about each tested medium is presented in Table 2. In order to prepare hemin solution, 0.5 g of hemin was dissolved in 10 mL of 1 N NaOH and afterwards distilled water was added to reach the volume of 100 mL. Further, vitamin K1 solution was prepared by adding 0.025 mL of vitamin K1 stock solution to 4.975 mL of 95% ethanol. The prepared solutions of hemin and vitamin K1 were used with a concentration of 0.0005% (w/v) and 0.0001% (w/v), respectively. In mGTS, Part III of this medium is a vitamin mixture, Sigma K3129, from Sigma–Aldrich (UK) with the stock solution of 100× that was used at a concentration of 0.5% (v/v).
| Species | Strain | Origin | Association with BV1 |
|---|---|---|---|
| Gardnerella sp. | UM2412 | Isolated from women diagnosed with BV | Often described |
| Atopobium vaginae | ATCC BAA-55T | Isolated from vaginal microbiota of a healthy woman (Jovita et al., 1999) | Often described |
| Lactobacillus iners | CCUG 28746T | Isolated from human urine (Falsen et al., 1999) | Commonly described |
| Mobiluncus curtisii | ATCC 35241T | Isolated from women with BV (Spiegel & Roberts, 1984) | Commonly described |
| Peptostreptococcus anaerobius | ATCC 27337T | Isolated from female genital tract (Ng et al., 1994) | Commonly described |
| Prevotella bivia | ATCC 29303T | Isolated from endometrium (Holdeman & Johnson, 1977) | Commonly described |
Notes:
| Culture medium | Composition | Supplementation | Abbreviation |
|---|---|---|---|
| Brain heart infusion broth (Liofilchem, Italy) | As described by the manufacturer | 2% (w/w) Gelatine (Liofilchem, Italy); 0.1% (w/w) Starch (Panreac, Spain); 0.5% (w/w) Yeast extract (Liofilchem, Italy) |
sBHI/sBHI.Aa1 |
| Brucella broth (Liofilchem, Italy) |
As described by the manufacturer | 0.0005% (w/v) Hemin (Sigma, China); 0.0001% (w/v) Vitamin K1 (Sigma, China) |
BHV/BHV.Aa1 |
| New York City III broth | 1.5% (w/v) Bacto proteose peptone no. 3 (BD, France); 0.5% (w/v) Glucose (Fisher Scientific, UK); 0.24% (w/v) HEPES (VWR, USA); 0.5% (w/v) NaCl (VWR, USA); 0.38% (w/v) Yeast extract (Liofilchem, Italy) |
10% (v/v) Inactivated horse serum (Biowest, France) |
NYC/NYC.Aa1 |
| Schaedler broth (Liofilchem, Italy) | As described by the manufacturer | – | SB/SB.Aa1 |
| Chemically defined medium simulating genital tract secretions (Stingley et al., 2014) | Part I: 0.35% NaCl; 0.15% KCl; 0.174% K2HPO4; 0.136% KH2PO4; 1.08% glucose; 0.05% cysteine HCl. Part II: 0.1% glycogen; 0.03% mucin; 0.02% tween 20; 0.05% urea; 0.0005% hemin; 0.0001% vitamin K1; 0.2% bovine serum albumin; 0.03% MgSO4; 0.004% NaHCO3; 0.1% sodium acetate; 0.005% MnCl2. Part III: 0.0005% biotin; 5.0% myo-inositol; 0.05% niacinamide; 0.05% pyridoxine HCI; 0.05% thiamin HCI; 0.05% D-calcium pantothenate; 0.05% folic acid; 0.001% p-aminobenzoic acid; 0.05% choline chloride; 0.01% riboflavin; 0.1% L-ascorbic acid; 0.0005% vitamin A (retinol); 0.0005% vitamin D (cholecalciferol); 0.001% vitamin B12. Part IV (amino acids): 0.032% alanine; 0.008% arginine; 0.076% aspartic acid; 0.036% glutamic acid; 0.04% glutamine; 0.02% glycine; 0.016% histidine; 0.012% isoleucine; 0.02% leucine; 0.02% lysine; 0.004% methionine; 0.004% phenylalanine; 0.028% proline; 0.012% serine; 0.012% threonine; 0.004% tryptophan; 0.02% tyrosine; 0.068% valine. Part V (UPI): 0.05% uracil; 0.01% sodium pyruvate; 0.02% inosine. |
– | mGTS2 |
Notes:
Planktonic growth assessment
For the evaluation of planktonic growth, the inoculums were prepared by transferring fresh bacterial colonies from CBA plates to 8 mL of each culture medium described above. The obtained bacterial suspensions were adjusted by optical density (OD) at 620 nm to 0.10 ± 0.05 (EZ Read 800 Plus; Biochrom, Cambridge, UK) and equally distributed in two sterile 15 mL falcon tubes (Orange Scientific, Braine-l’Alleud, Belgium) which were further incubated at 37 °C under anaerobic conditions for 48 h, as described above. Afterwards, planktonic growth was assessed by OD620nm. Growth was normalized as a fold difference between the final OD620nm and the starting OD (at time 0 h). The assays were repeated at least three times on separate days, with four technical replicates considered each time.
Biofilm formation and biomass quantification
Single-species biofilms of each tested species were initiated by inoculating bacterial suspensions of 48 h cultures adjusted to an OD620nm of 0.10 ± 0.05 in sterile 96-well tissue culture plates (Orange Scientific, Braine-l’Alleud, Belgium) and incubated for 72 h, at 37 °C under anaerobic conditions. To quantify the biofilm biomass, we used the crystal violet (CV) method, which is the most frequently employed approach for this purpose (Azeredo et al., 2017; Peeters, Nelis & Coenye, 2008). In brief, following 72 h of incubation, the biofilms were washed once with 0.9% (w/v) sodium chloride and allowed to air dry. After, the biofilms were fixed with 100% (v/v) methanol (Thermo Fisher Scientific, Waltham, MA, USA) for 20 min, and then stained with CV solution 1% (v/v) (Merck, Darmstadt, Germany) for 20 min. Subsequently, each well was washed twice with 1% (v/v) phosphate-buffered saline, and the bound CV was released with 33% (v/v) acetic acid (Thermo Fisher Scientific, Lenexa, KS, USA). To assess the biomass, the OD of the resulting solution was measured at 595 nm. Biofilm experiments were repeated at least three times with eight technical replicates.
Statistical analysis
The data were analyzed using the statistical package GraphPad Prism version 6 (La Jolla, CA, USA) by one-way ANOVA (Dunnett’s and Tukey’s multiple comparison tests) and two-way ANOVA (Sidak’s multiple comparisons test). Values with a p < 0.05 and p < 0.01 were considered statistically significant.
Results
Planktonic growth assays
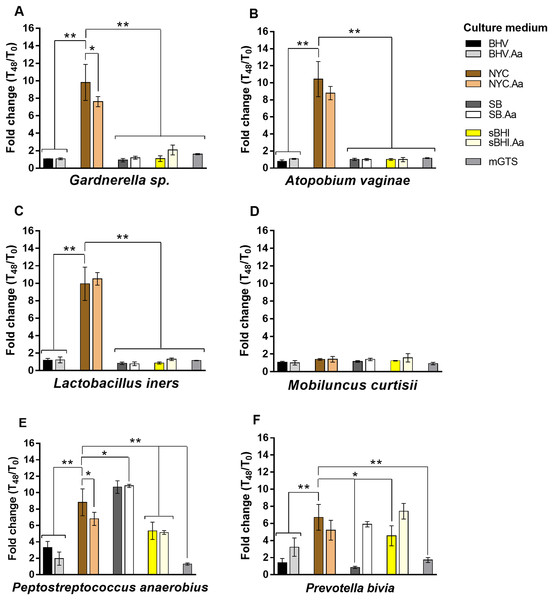
As shown in Fig. 1, BV-associated anaerobes had variable ability to grow planktonically in the tested culture media. Accordingly, P. anaerobius and P. bivia had higher metabolic flexibility and were able to grow in most of the tested media, while M. curtisii had more restrictive growth requirements and presented low levels of growth in all of them. Interestingly, NYC broth showed high levels of planktonic growth for the tested species, being overpassed only by NYC.Aa for L. iners, SB and SB.Aa for P. anaerobius, and by sBHI.Aa for P. bivia. The mGTS supported very low levels of bacterial growth, with only Gardnerella sp., P. anaerobius and P. bivia showing moderate levels of growth.
Figure 1: Fold change in planktonic growth of BV-associated bacteria in the nine different culture media relative to OD620nm values measured at T0 h.
(A) Experiments conducted with Gardnerella sp. (B) Experiments conducted with A. vaginae. (C) Experiments conducted with L. iners. (D) Experiments conducted with M. curtisii. (E) Experiments conducted with P. anaerobius. (F) Experiments conducted with P. bivia. Results represent the average ± the standard deviation of at least three independent experiments. Statistical analysis was performed using one-way ANOVA and Dunnett’s multiple comparisons test. Significant differences between NYC medium (our culture medium of choice) and other culture media are depicted with *p < 0.05 and **p < 0.01. Other statistical analysis is shown in Table S1.Since it was previously shown that L-ascorbic acid could enhance the growth of several anaerobic bacteria, including A. vaginae, Finegoldia magna, Fusobacterium necrophorum, Prevotella nigrescens, Ruminococcus gnavus and Solobacterium moorei (La Scola et al., 2014), we repeated the experiments with media supplemented with 0.1% (w/v) L-ascorbic acid. However, contrary to what was described before, the addition of 0.1% (w/v) L-ascorbic acid had a very variable effect on bacterial growth, with only 33.3% (n = 8; cut-off ≥ 1.25-fold change) of the total combinations tested yielding a significant increase in bacterial growth, while in 4.17% (n = 1; cut-off < 0.75-fold change) an inhibition of the growth was observed. In most of the tested combinations (n = 15; 0.75 ≤ fold change < 1.25), no effect was observed (Table S1). The most notable case was observed for P. bivia growth in SB, in which L-ascorbic acid increased almost seven-fold the growth rate.
Biofilm assays
Not surprisingly, we observed that similar to planktonic growth, biofilm formation was also strongly affected by the culture media composition, as depicted in Fig. 2. Importantly, there was not a direct relationship between higher planktonic growth and higher biofilm formation, which further confirms that the requirements for biofilm formation are distinct than the requirements for planktonic growth, as showed before for many other bacterial species (Alves et al., 2014; Heffernan, Murphy & Casey, 2009; Ripolles-Avila et al., 2018; Wijesinghe et al., 2019). Further differences between biofilm formation and planktonic growth were observed when adding L-ascorbic acid to the growth media, with 20.8% (n = 5) of the tested combinations species/growth medium resulting in a statistically significant decrease in the biofilm-forming capacity (p < 0.05) and 37.5% (n = 9) of the situations also presenting a visible biofilm reduction, however not statistically significant as can be seen in Table S2. Moreover, the addition of L-ascorbic acid to the culture media did not promote significantly increased biofilm formation in any of the combinations tested (Fig. 2).
Figure 2: Biofilm formation of BV-associated bacteria in the nine different culture media over a 72 h period.
Biofilm biomass was quantified using the crystal violet staining assay. (A) The total biofilm biomass formed by Gardnerella sp. in the tested culture media. (B) The total biofilm biomass formed by A. vaginae. (C) The total biofilm biomass formed by L. iners. (D) The total biofilm biomass formed by M. curtisii. (E) The total biofilm biomass formed by P. anaerobius. (F) The total biofilm biomass formed by P. bivia. Results are expressed as the average ± standard deviation of at least three independent experiments performed with eight technical replicates. Statistical analysis was performed using one-way ANOVA and Dunnett’s multiple comparisons test. Significant differences between biofilm biomass formed in NYC medium (our culture medium of choice) and other culture media are represented with *p < 0.05 and **p < 0.01. Other statistical analysis is shown in Table S2.Discussion
Despite the fact that BV is an increasingly important health problem, there is a lack of studies addressing multi-species interactions that might occur during BV and their role in the development of this infection. Most attempts to understand the microbiology behind BV have been focused mainly on Gardnerella spp., perhaps because this species has long been associated with BV development (Gardner & Dukes, 1955; Swidsinski et al., 2005) and it has been now hypothesized that this microorganism is the initial colonizer of the vaginal epithelium, being able to establish an early biofilm structure to which other BV-associated species can attach (Muzny et al., 2019). However, the role of these species in the development and progress of BV is still poorly understood and therefore, more studies are needed to unravel this matter.
We showed before that BV-associated species had different abilities to grow as biofilms, and this was strongly dependent on the growth media (Alves et al., 2014). As such, the first step in facilitating BV multi-species biofilm studies is to determine optimal culture medium conditions suitable for multiple BV-associates species, considering to further investigate the interactions that might exist between them in BV multi-species biofilms and their implications in BV process.
Although sBHI has been widely used as a medium that supports Gardnerella spp. growth (Algburi, Volski & Chikindas, 2015; Gottschick et al., 2016; Harwich et al., 2010; Machado, Palmeira-de-Oliveira & Cerca, 2015; Patterson et al., 2010; Turovskiy et al., 2012; Weeks et al., 2019), it did not facilitate the planktonic growth or biofilm formation for some of the tested species, including A. vaginae, L. iners and M. curtisii. The same was observed for these three species in SB medium, even though the manufacturer describes it as a medium suitable for the cultivation of anaerobic microorganisms, providing them an important amount of amino acids, nitrogen, vitamins as well as the energy necessary for growth. In an early study, after evaluating nine broth media in varied CO2 atmospheres for their ability to support the growth of anaerobic bacteria including Bacteroides fragilis subspecies fragilis, Peptostreptococcus CDC group 2, Eubacterium alactolyticum and Clostridium perfringens, Stalons, Thornsberry & Dowell (1974) found that SB in an atmosphere of 5% CO2, 10% hydrogen and 85% nitrogen exhibited the fastest and highest growth response. However, in our in vitro conditions, we obtained high levels of planktonic growth only for P. anaerobius, probably because this medium is not appropriate for the growth of all species of anaerobic microorganisms. Still, SB was a good medium to support in vitro biofilm formation with high levels of the biomass for Gardnerella sp., P. anaerobius and P. bivia. Interestingly, Gardnerella sp. and P. bivia showed in SB the lowest levels of planktonic growth, but the highest biofilm formation ability. As mentioned, SB is a complex medium and perhaps the presence of certain growth factors determined these two anaerobes to turn on the expression of biofilm-related genes. Another of the tested media, BHV, also described by the manufacturer as suitable for the cultivation of anaerobes, was not appropriate for the growth and biofilm formation of the tested species, with the exception of planktonic growth by P. anaerobius (Fig. 1).
Interestingly, NYC facilitated the planktonic growth of all tested species, despite M. curtisii presented a very slow growth rate. Nevertheless, even M. curtisii was able to form high biofilm biomass in this medium. In fact, together with A. vaginae and L. iners, significant biofilm formation was only detected in NYC (Fig. 2). A particularity of NYC medium, compared to the other tested media, is the presence of proteose peptone no. 3, which has been described by the manufacturer as offering high nutritional benefits to fastidious anaerobic species by providing the necessary amount of nitrogen, carbon, amino acids and essential growth factors. To assess if, in fact, the enhancement of biofilm formation in NYC was mainly due to the presence of proteose peptone no. 3, we carried out an experiment by evaluating the biofilm-formation ability of the six tested bacterial species in the original recipe of NYC versus an altered version of NYC [with regular peptone from meat (Acros Organics, UK) replacing the proteose peptone no. 3]. Interestingly, while we did find that proteose peptone no. 3 was essential to the biofilm formation by M. curtisii, no significant differences were found for the other species (Fig. S1), which suggests that NYCʼs ability to enhance biofilm formation is not only related to the presence of proteose peptone no. 3.
Besides the commercially available media, we also tested a chemically defined medium that simulates the genital tract fluid, mGTS (Stingley et al., 2014). Since mGTS is a minimal medium without rich nutrient sources, it was not surprising that the growth of the tested BV-associated species was negligent or very slow in this medium. Nevertheless, biofilm formation by Gardnerella sp. and P. anaerobius was significant under mGTS, further being confirmed that biofilm formation requires specific conditions, different from planktonic growth.
We also tested another variable in our growth conditions optimization. The addition of L-ascorbic acid had the advantage of reducing the oxidation potential of the growth media by removing the oxygen (La Scola et al., 2014). However, the effect of adding L-ascorbic acid was very variable, depending not only on the bacterial species but also on the respective growth media. Nevertheless, there was a tendency to slightly or highly suppress biofilm formation. Interestingly, the inhibition of biofilm formation by ascorbic acid has been described before in biofilms of Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa (Pandit et al., 2017) as well as of methicillin-resistant Staphylococcus aureus (Mirani et al., 2018). It should be noted that at higher concentrations, L-ascorbic acid has been reported as a possible adjuvant for antibiotic treatment of BV, playing a role in maintaining a low vaginal pH, which favors the recolonization of the vaginal environment with lactic acid-producing bacteria, decreasing, thereby, the risk of BV recurrence (Krasnopolsky et al., 2013; Polatti et al., 2006). Our data further expand these previous findings by demonstrating that, while sometimes favoring planktonic growth, L-ascorbic acid often impairs biofilm formation.
A limitation of this study was the fact that we only tested a yet unidentified Gardnerella sp. isolate, but at least three new species have been recently reported. Previously, we assessed biofilm formation by seven clinical isolates from BV-women and seven from healthy microbiota and found no significant differences between the ability to form biofilms by the 2 groups, using different growth media (Castro et al., 2015). We now know that from those 14 isolates, some belong to G. vaginalis, G. leopoldii, G. piotii and G. swidsinskii (Castro et al., 2020). As such, we hypothesized that the four Gardnerella species would have similar biofilm formation abilities in our growth medium of choice: NYC. To test this hypothesis, we selected one isolate of each species, previously found to form similar biofilms in sBHI (Castro et al., 2020; Vaneechoutte et al., 2019), and compared its biofilms with NYC medium. As shown in Fig. S2, all the tested species had a similar biofilm-formation ability as compared to Gardnerella sp. UM241, with G. leopoldii showing a slight decrease in biomass, but well within the expected variation found in different Gardnerella strains (Castro et al., 2015).
Conclusions
Overall, our work has shed new light on the optimal conditions required for in vitro growth and biofilm formation of bacteria associated with BV. Although we tested nine different growth conditions, including a medium simulating genital tract secretions (mGTS), none of them is able to account for all growth factors present in the vaginal environment, including components of the host immune system, that are known to interfere in bacterial growth (Castro, Jefferson & Cerca, 2018). Nevertheless, this work highlighted that under the appropriate in vitro conditions, some of the most common species found in BV can form single-species biofilms, contrary to what was shown before (Castro et al., 2020; Patterson et al., 2010). NYC medium revealed to be an ideal candidate for future studies addressing multi-species biofilm formation since this growth medium allowed significant levels of single-species biofilm formation. Understanding microbial interactions that occur during BV development is crucial for the development of novel antimicrobial strategies, and future work will help to clarify some of these crucial interactions in multi-species biofilms.
Supplemental Information
Biofilm formation of BV-associated bacteria in NYC (with proteose peptone no. 3) and altered version of NYC (with peptone from meat) over a 72 h period.
Biofilm biomass was quantified using the crystal violet staining assay. Results represent the average ± the standard deviation of three independent experiments performed with eight technical replicates. Statistical analysis was performed using two-way ANOVA and Sidak’s multiple comparisons test. No significant difference was found between biofilm-formation ability of BV-associated bacteria in the two tested culture media.
Biofilm formation of G. piotii, G. leopoldii, G. vaginalis, and G. swidsinskii in NYC and sBHI over a 72 h period.
Biofilm biomass was quantified using the crystal violet staining method. Results represent the average ± the standard deviation of three independent experiments performed with eight technical replicates. Statistical analysis was performed using two-way ANOVA and Sidak’s multiple comparisons test. Significant differences between biofilm biomass formed in NYC and sBHI are represented with *p < 0.05 and **p < 0.01.
Fold change in planktonic growth of BV-associated bacteria in the nine different culture media relative to OD620nm values measured at T0h.
1 Statistical differences between bacterial planktonic growths in different culture media were analyzed with one-way ANOVA and Tukey’s multiple comparisons test, p < 0.05. a Statistical significance when comparing with BHV, b when comparing with BHV.Aa, c when comparing with NYC, d when comparing with NYC.Aa, e when comparing with SB, f when comparing with SB.Aa, g when comparing with sBHI, h when comparing with sBHI.Aa. 2 The effect of L-ascorbic acid on bacterial growth is presented as fold change relative to the growth in the medium without L-ascorbic acid (fold change = 1, control). This effect was classified as inhibitory (cut-off < 0.75 – fold change), neutral (0.75 ≤ fold change < 1.25), and stimulatory (cut-off ≥ 1.25 – fold change).
Biofilm formation of BV-associated bacteria in the nine different culture media over a 72 h period1.
1 Biofilm biomass was quantified using the crystal violet staining method and the resulting solution was assessed by optical density (OD) at 595 nm. 2 Statistical differences between bacterial planktonic growths in different culture media were analyzed with one-way ANOVA and Tukey’s multiple comparisons test, p < 0.05. a Statistical significance when comparing with BHV, b when comparing with BHV.Aa, c when comparing with NYC, d when comparing with NYC.Aa, e when comparing with SB, f when comparing with SB.Aa, g when comparing with sBHI.Aa.