WO2007039615A1 - New n-alkyl-heterocyclyl carboxamide derivatives - Google Patents
New n-alkyl-heterocyclyl carboxamide derivatives Download PDFInfo
- Publication number
- WO2007039615A1 WO2007039615A1 PCT/EP2006/067013 EP2006067013W WO2007039615A1 WO 2007039615 A1 WO2007039615 A1 WO 2007039615A1 EP 2006067013 W EP2006067013 W EP 2006067013W WO 2007039615 A1 WO2007039615 A1 WO 2007039615A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- compound
- carboxamide
- dimethylhexyl
- general formula
- Prior art date
Links
- 0 C*(C)C1CCCC1 Chemical compound C*(C)C1CCCC1 0.000 description 4
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/30—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members
- C07D207/34—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/08—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings with oxygen as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/10—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings with sulfur as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/36—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom five-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/74—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,3
- A01N43/78—1,3-Thiazoles; Hydrogenated 1,3-thiazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/02—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings
- C07D231/10—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D231/14—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/02—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings
- C07D277/20—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/38—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D307/54—Radicals substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/56—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/28—Halogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/38—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
Definitions
- the present invention relates to novel N-alkyl-heterocyclyl carboxamide derivatives, their process of preparation, their use as fungicides, particularly in the form of fungicidal compositions, and methods for the control of phytopathogenic fungi of plants using these compounds or their compositions.
- Japanese patent application JP 9176124 discloses pyrazolin carboxamide derivatives of general formula encompassing the compounds according to the present invention, and their use as fungicide. However, compounds according to the present invention are not disclosed in that patent application.
- US patent application US 5,240,951 discloses isothiazole carboxamide derivatives of general formula encompassing the compounds according to the present invention, and their use as fungicide. However, compounds according to the present invention are not disclosed in that patent application.
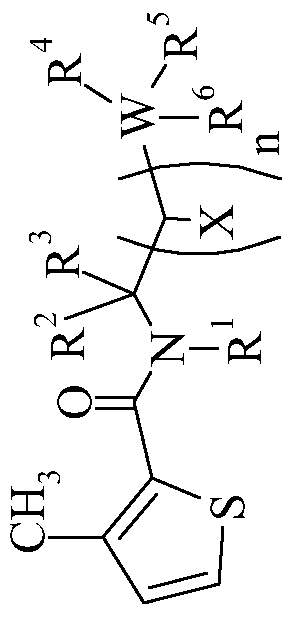
- the present invention relates to a N-alkyl-heterocyclyl carboxamide derivative of general formula (I)
- Het represents 5-membered non-fused heterocycle with one, two or three heteroatoms which may be the same or different, Het being linked by a carbon atom and being at least substituted in ortho position;
- R 1 is chosen as being a hydrogen atom, a Ci-C 6 -alkyl group or a C 3 -C 7 - cycloalkyl;
- - R is chosen as being a Ci-C 6 -alkyl group
- - R 3 is chosen as being a hydrogen atom or a Ci-C ⁇ -alkyl group
- each X is chosen, independently of the others, as being a hydrogen atom or a Ci-Ce-alkyl group;
- - W represents a carbon atom or a silicon atom
- R 4 , R 5 and R 6 are chosen, independently of each other, as being a hydrogen atom, a halogen atom, or a Ci-C 6 -alkyl group, at least two of the substituents R 4 , R 5 and R 6 being different from a hydrogen atom; or two of the substituents R 4 , R 5 and R 6 may together form a 3-, 4-, 5- or 6- membered non aromatic cycle optionally substituted with a Ci-C6-alkyl group; as well as its salts, N-oxydes, metallic complexes, metalloidic complexes and optically active isomers with the exception of :
- - halogen means fluorine, bromine, chlorine or iodine.
- any of the compound of the present invention can exist in one or more optical or chiral isomer forms depending on the number of asymmetric centres in the compound.
- the invention thus relates equally to all the optical isomers and to their racemic or scalemic mixtures (the term "scalemic” denotes a mixture of enantiomers in different proportions), and to the mixtures of all the possible stereoisomers, in all proportions.
- the diastereoisomers and/or the optical isomers can be separated according to the methods which are known per se by the man ordinary skilled in the art.
- Any of the compound of the present invention can also exist in one or more geometric isomer forms depending on the number of double bonds in the compound.
- the invention thus relates equally to all geometric isomers and to all possible mixtures, in all proportions.
- the geometric isomers can be separated according to general methods, which are known per se by the man ordinary skilled in the art.
- Het of the compound of general formula (I) is a 5-membered non-fused heterocycle with one, two or three heteroatoms which may be the same or different, Het being linked by a carbon atom and being at least substituted in ortho position.
- the present invention relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I) in which the different characteristics may be chosen alone or in combination as being :
- Het is chosen as being 2-furan, 3-furan, 4,5-dihydro-3-furan, 2-thiophene, 3-thiophene, 2-pyrrole, 3-pyrrole, 5-oxazole, 4-oxazole, 5-thiazole, A- thiazole, 5-pyrazole, 4-pyrazole, 3-pyrazole, 3-isoxazole, 4-isoxazole, 5-isoxazole, 3- isothiazole, 4-1,2,3-triazole, 4-thiadiazole or 5-thiadiazole;
- each substituent is chosen, independently of the others, as being a hydrogen atom, a halogen atom, an amino group, a cyano group, a Ci-C4-alkylamino, a di-(Ci-C4-alkyl)amino, a Ci-C4-alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms, a Ci-C 4 -halogenoalkoxy having 1 to 5 halogen atoms, a nitro group, a Ci-C4-alkyloxy, a Ci-C4-alkylthio, a C1-C4- alkylsulphonyl, a phenyl optionally substituted by a halogen atom or a Ci-C 4 -alkyl or a pyridyl optionally substituted by a halogen atom or a Ci-C 4 atom,
- the substituent in ortho position of the "Het” moiety is chosen as being a halogen atom, a Ci-C 4 -alkyl, a Ci- C 4 -halogenoalkyl having 1 to 5 halogen atoms, a Ci-C 4 -halogenoalkoxy having 1 to 5 halogen atoms or a Ci-C4-alkyloxy;
- Specific examples of Het moiety include :
- Het represents a heterocycle of the general formula (Het-1)
- R 7 and R 8 may be the same or different and may be a hydrogen atom, a halogen atom, an amino group, a nitro group, a Ci-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms;
- R 9 may be a halogen atom, a nitro group, a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms.
- Het represents a heterocycle of the general formula (Het-2)
- - R 10 may be a hydrogen atom, a halogen atom , a Ci-C 4 -alkyl or a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms;
- R 11 and R 12 may be the same or different and may be a hydrogen atom, a halogen atom, an amino group, a Ci-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms; provided that the R 10 and R 12 are not both a hydrogen atom.
- Het represents a heterocycle of the general formula (Het-3)
- - R 13 may be a halogen atom, a Ci-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms; and - R 14 may be a hydrogen atom, a Ci-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having
- Het represents a heterocycle of the general formula (Het-4)
- R 15 and R 16 may be the same or different and may be a hydrogen atom, a halogen atom, a Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms, a
- R 17 may be a halogen atom, a cyano group, a Ci-C4-alkyl, a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms or a Ci-C 4 -halogenoalkoxy having 1 to 5 halogen atoms.
- Het represents a heterocycle of the general formula (Het-5)
- R - R , 18 and i ⁇ R19 may be the same or different and may be a hydrogen atom, a halogen atom, a Ci-C 4 -alkyl, a Ci-C 4 -alkyloxy or a Ci-C 4 -halogenoalkyl having 1 to
- R 20 may be a hydrogen atom, a halogen atom, a Ci-C 4 -alkyl or a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms; provided that the R 19 and R 20 are not both a hydrogen atom.
- Het represents a heterocycle of the general formula (Het-6)
- R 21 may be a hydrogen atom, a halogen atom, a cyano group, a Ci-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms;
- R 22 and R 24 may be the same or different and may be a hydrogen atom, a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms;
- R 23 may be a hydrogen atom, a cyano group, a Ci-C 4 -alkyl, a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms, a Ci-C 4 -alkoxy-Ci-C 4 -alkyl, a hydroxy- Ci-C 4 -alkyl, a Ci-C 4 -alkylsulphonyl, a di(Ci-C 4 -alkyl)aminosulphonyl, a Ci-C 6 - alkylcarbonyl, a phenylsulphonyl optionally substituted by a halogen atom or a Ci- C 4 -alkyl, or a benzoyl optionally substituted by a halogen atom or a Ci-C 4 -alkyl; provided that the R 21 and R 24 are not both a hydrogen atom.
- Het represents a heterocycle of the general formula (Het-7)
- R 25 may be a hydrogen atom, a cyano group, a Ci-C 4 -alkyl, a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms, a Ci-C 4 -alkoxy-Ci-C 4 -alkyl, a hydroxy- Ci-C 4 -alkyl, a Ci-C 4 -alkylsulphonyl, a di(Ci-C 4 -alkyl)aminosulphonyl, a Ci-C 6 - alkylcarbonyl, a phenylsulphonyl optionally substituted by a halogen atom or a Ci- C 4 -alkyl, or a benzoyl optionally substituted by a halogen atom or a Ci-C 4 -alkyl; and
- R 26 , R 27 and R 28 may be the same or different and may be a hydrogen atom, a halogen atom, a cyano group, a Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms or a Ci-C 4 -alkylcarbonyl; provided that R 25 and R 28 are not both a hydrogen atom.
- Het represents a heterocycle of the general formula (Het-8)
- R 30 may be a halogen atom, a Ci-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms.
- Het represents a heterocycle of the general formula (Het-9)
- R 31 may be a hydrogen atom or a Ci-C 4 -alkyl
- R 32 may be a halogen atom, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms or a phenyl optionally substituted by a halogen atom or a C 1 -C 4 - alkyl.
- Het represents a heterocycle of the general formula (Het- 10)
- R 33 may be a hydrogen atom, a halogen atom, an amino group, a cyano group, a Ci-C4-alkylamino, a di-(Ci-C4-alkyl)amino, a Ci-C4-alkyl, a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms or a phenyl optionally substituted by a halogen atom or a d-C ⁇ alkyl; and
- R 34 may be a halogen atom, a Q-Gralkyl or a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms.
- Het represents a heterocycle of the general formula (Het-11)
- - R 35 may be a hydrogen atom, a halogen atom, an amino group, a cyano group, a Ci-C4-alkylamino, a di-(Ci-C4-alkyl)amino, a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms; and
- -R 36 may be a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms.
- Het represents a heterocycle of the general formula (Het-12)
- R 37 may be a halogen atom, a cyano group, a nitro group, a Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms, a C 3 -C 6 -cycloalkyl, a C 1 -C 4 - alkoxy, a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms, a Ci-C4-alkylthio, a Ci-C4-halogenoalkylthio having 1 to 5 halogen atoms, an aminocarbonyl group or an aminocarbonyl-Ci-C 4 -alkyl;
- R 38 may be a hydrogen atom, a halogen atom, a cyano group, a nitro group, a Ci-C4-alkyl, a Ci-C4-alkoxy or a Ci-C4-alkylthio;
- R 39 may be a hydrogen atom, a phenyl, a Ci-C 4 -alkyl, a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms, a hydroxy-Ci-C 4 -alkyl, a C 2 -C 6 -alkenyl, a C 3 -C 6 -cycloalkyl, a Ci-C 4 -alkylthio-Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkylthio-Ci-C 4 - alkyl having 1 to 5 halogen atoms, a Ci-C 4 -alkoxy-Ci-C 4 -alkyl or a C 1 -C 4 - halogenoalkoxy-Ci-C 4 -alkyl having 1 to 5 halogen atoms.
- Het represents a heterocycle of the general formula (Het- 13)
- R 40 may be a hydrogen atom, a halogen atom, a cyano group, a nitro group, a Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms, a C 3 -C 6 - cycloalkyl, a Ci-C4-alkoxy, a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms, a Ci-C 4 -alkylthio, a Ci-C 4 -halogenoalkylthio having 1 to 5 halogen atoms, an aminocarbonyl or an aminocarbonyl-Ci-C 4 -alkyl;
- R 41 may be a hydrogen atom, a halogen atom, a cyano group, a Ci-C 4 -alkyl, a Ci-C 4 -alkoxy, a Ci-C 4 -halogenoalkoxy having 1 to 5 halogen atoms or a C 1 -C 4 - alkylthio; and
- R 42 may be a hydrogen atom, a Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms, a hydroxy-Ci-C 4 -alkyl, a C 2 -C 6 -alkenyl, a C 3 -C 6 -cycloalkyl, a Ci-C 4 -alkylthio-Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkylthio-Ci-C 4 -alkyl having 1 to 5 halogen atoms, a Ci-C 4 -alkoxy-Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkoxy-Ci-C 4 -alkyl having 1 to 5 halogen atoms or a phenyl optionally substituted by a halogen atom, a Ci-C 4 -alkyl, a Ci-C 4
- Het represents a heterocycle of the general formula (Het-14)
- -R 43 may be a hydrogen atom, a halogen atom, a cyano group, a nitro group, a Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms, a C 3 -C 6 -cycloalkyl, a Ci-C 4 -alkoxy, a Ci-C 4 -halogenoalkoxy having 1 to 5 halogen atoms, a C 1 -C 4 - alkylthio, a Ci-C 4 -halogenoalkylthio having 1 to 5 halogen atoms, an aminocarbonyl, or an aminocarbonyl-Ci-C 4 -alkyl;
- R 44 may be a hydrogen atom, a halogen atom, a cyano group, a Ci-C 4 -alkyl, a Ci-C 4 -alkoxy, a Ci-C 4 -alkylthio or a Ci-C 4 -halogenoalky having 1 to 5 halogen atoms;
- R 45 may be a hydrogen atom, a phenyl, a benzyl, a Ci-C 4 -alkyl, a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms, a hydroxy-Ci-C 4 -alkyl, a C 2 -C 6 -alkenyl, a C 3 -C 6 -cycloalkyl, a Ci-C 4 -alkylthio-Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkylthio-Ci-C 4 - alkyl having 1 to 5 halogen atoms, a Ci-C 4 -alkoxy-Ci-C 4 -alkyl, a C 1 -C 4 - halogenoalkoxy-Ci-C 4 -alkyl having 1 to 5 halogen atoms; provided that R 44 and R 45 are not both a hydrogen atom. He
- - R 46 may be a hydrogen atom, a halogen atom, a C-C 4 -alkyl or a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms; and - R 47 may be a halogen atom, a C-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms.
- Het represents a heterocycle of the general formula (Het- 16)
- R 48 and R 49 may be the same or different and may be a hydrogen atom, a halogen atom, a Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms, a phenyl optionally substituted by a halogen atom or a Ci-C 4 -alkyl, or a heterocyclyl optionally substituted by a halogen atom or a Ci-C 4 -alkyl; provided that R 48 and R 498 are not both a hydrogen atom.
- Het represents a heterocycle of the general formula (Het- 17)
- - R 50 may be a halogen atom, a Ci-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having
- - R > 51 may be a halogen atom, a Ci-C 4 -alkyl or a Ci-C 4 -halogenoalkyl having
- Het represents a heterocycle of the general formula (Het-18)
- R 52 may uc a iicu ⁇ gcii ciLUii, a Ci-C 4 -alkyl or a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms.
- Het represents a heterocycle of the general formula (Het- 19)
- R 53 may be a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms;
- R 54 may be a hydrogen atom, a Ci-C 4 -alkyl, a Ci-C 4 -halogenoalkyl having 1 to 5 halogen atoms, or a phenyl optionally substituted by a halogen atom or a C 1 -C 4 - alkyl.
- Het represents a heterocycle of the general formula (Het-20)
- R 55 may be a halogen atom, a Ci-C 4 -alkyl or a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms.
- Het may represent a heterocycle of the general formula (Het-21)
- R 56 may be a halogen atom, a Ci-C 4 -alkyl or a C 1 -C 4 - halogenoalkyl having 1 to 5 halogen atoms.
- the nitrogen atom of the carboxamide moiety of the compound of formula (I) is substituted by R 1 , R 1 being a hydrogen atom or a C3-C7-cycloalkyl.
- the C 3 -C 7 -CyC loalkyl is cyclopropyl.
- the first carbon atom of the alkyl chain of the compound of formula (I) is substituted by R 2 , R 2 being as defined above.
- the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I) in which R 2 may be chosen as being a Ci-C 3 alkyl.
- R is a methyl group.
- the alkyl chain of the compound of formula (I) further comprises n carbon atoms, each of which being substituted by R 3 , R 3 being as defined above.
- the present invention also relates to N-alkyl- heterocyclyl carboxamide derivative of general formula (I) in which the different characteristics may be chosen alone or in combination as being :
- n 3;
- R 3 is a methyl group.
- the last carbon atom of the alkyl chain of the compound of formula (I) is substituted by R 4 , R 5 and R 6 , R 4 , R 5 and R 6 being as defined above.
- the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I) in which R 4 , R 5 and R 6 may be chosen, independently of each other as being a hydrogen atom or a methyl group.
- the alkyl chain of the compound of formula (I) contains from 2 to 4 carbon atoms, each of which is substituted by a substituent X which may the same or different.
- the present invention relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I) in which X is chosen as being a hydrogen atom or a methyl group.
- the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I-a) in which :
- R 1 , R 2 , R 3 , R 4 , R 5 and R 6 are as defined above;
- - X 1 and X 2 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
- the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I-b)
- R 1 , R 2 , R 3 , R 4 , R 5 and R 6 are as defined above;
- X 1 , X 2 and X 3 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
- the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I-c)
- R 1 , R 2 , R 3 , R 4 , R 5 and R 6 are as defined above;
- X 1 , X 2 , X 3 and X 4 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
- the present invention also relates to a process for the preparation of the compound of general formula (I).
- a process for the preparation of compound of general formula (I) as defined above which comprises reacting an alkyl-amine derivative of general formula (II) or one of its salt
- -Het is as defined above; and - L 1 is a leaving group chosen as being a halogen atom, a hydroxyl group, -
- OR 54 , -OCOR 54 , R 54 being a Ci-C 6 alkyl, a Ci-C 6 haloalkyl, a benzyl, 4- methoxybenzyl, pentafluorophenyl or a group of formula j? ;
- the process according to the present invention is conducted in the presence of a catalyst.
- Suitable catalyst may be chosen as being 4-dimethyl-aminopyridine, 1- hydroxy-benzotriazole or dimethylformamide.
- L 1 is a hydroxy group
- the process according to the present invention is conducted in the presence of condensing agent.
- Suitable condensing agent may be chosen as being acid halide former, such as phosgene, phosphorous tribromide, phosphorous trichloride, phosphorous pentachloride, phosphorous trichloride oxide or thionyl chloride; anhydride former, such as ethyl chloroformate, methyl chloroformate, isopropyl chloroformate, isobutyl chloroformate or methanesulfonyl- chloride; carbodiimides, such as N,N'-dicyclohexylcarbodiimide (DCC) or other customary condensing agents, such as phosphorous pentoxide, polyphosphoric acid, N,N'-carbonyl-diimidazole, 2-ethoxy-N-ethoxycarbonyl- 1 ,2-dihydroquinoline
- acid halide former such as phosgene, phosphorous tribromide, phosphorous trichloride,
- EEDQ triphenylphosphine/tetrachloromethane
- - L 2 is a leaving group chosen as being a halogen atom, a 4- methyl phenylsulfonyloxy or a methylsulfonyloxy;
- R 1 a is a Ci-C 6 -alkyl group or a C 3 -Cy-cycloalkyl; comprising the reaction of a compound of general formula (Ib) with a compound of general formula (VIII) to provide a compound of general formula (Ia).
- amine derivatives of general formula (II) may be prepared by different processes.
- One example (A) of such a process may be when :
- R 1 , R 2 , X, n, W, R 4 , R 5 and R 6 are as defined above;
- the amine derivative of general formula (II) may be prepared according to a process which comprises :
- reaction scheme A-I a first step according to reaction scheme A-I :
- An other example (B) of such a process may be when :
- R 2 , R 3 , X, n, W, R 4 , R 5 , R 6 are as defined above;
- the amine derivative of general formula (II) may be prepared according to a process which comprises :
- R 2 , R 3 , X, n, W, R 4 , R 5 and R 6 are as defined above;
- - U is a leaving group chosen as being a halogen atom, a Ci-C 6 alkylsulfonate or a Ci-C 6 haloalkylsulfonate; comprising the nucleophilic substitution of a compound of general formula (VI) by a phtalimide salt to produce a compound of general formula (VII); - a second step according to reaction scheme B-2 : Scheme B-2
- R 2 , R 3 , X, n, W, R 4 , R 5 , R 6 are as defined above; comprising the de-protection of a compound of general formula (VII) by reacting it with hydrazine hydrate or a hydrazine salt to provide an amine derivative of general formula (lib) or one of its salt;
- the compound of general formula (II) used as an intermediate for the preparation of compound of general formula (I) is novel. Therefore, the present invention also relates to novel intermediate compound useful for the preparation of compound of general formula (I). Thus, according to the present invention, there is provided a compound of general formula (II)
- R 1 , R 2 , R 3 , X, n, W, R 4 , R 5 and R 6 are as defined above
- the present invention also relates to a fungicidal composition
- a fungicidal composition comprising an effective amount of an active material of general formula (I).
- a fungicidal composition comprising, as an active ingredient, an effective amount of a compound of general formula (I) as defined above and an agriculturally acceptable support, carrier or filler.
- support denotes a natural or synthetic, organic or inorganic material with which the active material is combined to make it easier to apply, notably to the parts of the plant. This support is thus generally inert and should be agriculturally acceptable.
- the support may be a solid or a liquid.
- suitable supports include clays, natural or synthetic silicates, silica, resins, waxes, solid fertilisers, water, alcohols, in particular butanol, organic solvents, mineral and plant oils and derivatives thereof. Mixtures of such supports may also be used.
- the composition may also comprise additional components.
- the composition may further comprise a surfactant.
- the surfactant can be an emulsifier, a dispersing agent or a wetting agent of ionic or non-ionic type or a mixture of such surfactants.
- polyacrylic acid salts lignosulphonic acid salts, phenolsulphonic or naphthalenesulphonic acid salts
- polycondensates of ethylene oxide with fatty alcohols or with fatty acids or with fatty amines substituted phenols (in particular alkylphenols or
- the presence of at least one surfactant is generally essential when the active material and/or the inert support are water- insoluble and when the vector agent for the application is water.
- surfactant content may be comprised between 5% and 40% by weight of the composition.
- additional components may also be included, e.g. protective colloids, adhesives, thickeners, thixotropic agents, penetration agents, stabilisers, sequestering agents.
- the active materials can be combined with any solid or liquid additive, which complies with the usual formulation techniques.
- composition according to the invention may contain from 0.05 to 99% (by weight) of active material, preferably 10 to 70% by weight.
- compositions according to the present invention can be used in various forms such as aerosol dispenser, capsule suspension, cold fogging concentrate, dustable powder, emulsifiable concentrate, emulsion oil in water, emulsion water in oil, encapsulated granule, fine granule, fiowable concentrate for seed treatment, gas (under pressure),gas generating product, granule, hot fogging concentrate, macrogranule, microgranule, oil dispersible powder, oil miscible fiowable concentrate, oil miscible liquid, paste, plant rodlet, powder for dry seed treatment, seed coated with a pesticide, soluble concentrate, soluble powder, solution for seed treatment, suspension concentrate (fiowable concentrate), ultra low volume (ulv) liquid, ultra low volume (ulv) suspension, water dispersible granules or tablets, water dispersible powder for slurry treatment, water soluble granules or tablets, water soluble powder for seed treatment and wettable powder.
- aerosol dispenser capsule suspension, cold fogging concentrate
- dustable powder
- compositions include not only compositions which are ready to be applied to the plant or seed to be treated by means of a suitable device, such as a spraying or dusting device, but also concentrated commercial compositions which must be diluted before application to the crop.
- the compounds of the invention can also be mixed with one or more insecticides, fungicides, bactericides, attractant acaricides or pheromones or other compounds with biological activity. The mixtures thus obtained have a broadened spectrum of activity. The mixtures with other fungicides are particularly advantageous.
- fungicide mixing partners may be selected in the following lists :
- a compound capable to inhibit the nucleic acid synthesis like benalaxyl, benalaxyl-M, bupirimate, chiralaxyl, clozylacon, dimethirimol, ethirimol, furalaxyl, hymexazol, metalaxyl-M, ofurace, oxadixyl, oxolinic acid ;
- a compound capable to inhibit the mitosis and cell division like benomyl, carbendazim, diethofencarb, fuberidazole, pencycuron, thiabendazole thiophanate- methyl, zoxamide;
- a compound capable to inhibit the respiration for example as Cl-respiration inhibitor like diflumetorim; as Cll-respiration inhibitor like boscalid, carboxin, fenfuram, flutolanil, furametpyr, mepronil, oxycarboxine, penthiopyrad, thifiuzamide; as CHI-respiration inhibitor like azoxystrobin, cyazofamid, dimoxystrobin, enestrobin, famoxadone, fenamidone, fluoxastrobin, kresoxim-methyl, metominostrobin, orysastrobin, pyraclostrobin, picoxystrobin, trifloxystrobin; 4) a compound capable of to act as an uncoupler like dinocap, fluazinam;
- a compound capable to inhibit ATP production like fentin acetate, fentin chloride, fentin hydroxide, silthiofam;
- a compound capable to inhibit lipid and membrane synthesis like chlozolinate, iprodione, procymidone, vinclozolin, pyrazophos, edifenphos, iprobenfos (IBP), isoprothiolane, tolclofos-methyl, biphenyl, iodocarb, propamocarb, propamocarb-hydrochloride;
- a compound capable to inhibit ergosterol biosynthesis like fenhexamid, azaconazole, bitertanol, bromuconazole, cyproconazole, diclobutrazole, difenoconazole, diniconazole, diniconazole-M, epoxiconazole, etaconazole, fenbuconazole, fluquinconazole, fiusilazole, fiutriafol, furconazole, furconazole-cis, hexaconazole, imibenconazole, ipconazole, metconazole, myclobutanil, paclobutrazol, penconazole, propiconazole, prothioconazole, simeconazole, tebuconazole, tetraconazole, triadimefon, triadimenol, triticonazole, uniconazole, voriconazole, imazalil
- a compound capable to inhibit cell wall synthesis like benthiavalicarb, bialaphos, dimethomorph, flumorph, iprovalicarb, polyoxins, polyoxorim, validamycin A; 11) a compound capable to inhibit melanine biosynthesis like carpropamid, diclocymet, fenoxanil, phtalide, pyroquilon, tricyclazole;
- a compound capable to have a multisite action like captafol, captan, chlorothalonil, copper preparations such as copper hydroxide, copper naphthenate, copper oxychloride, copper sulphate, copper oxide, oxine-copper and Bordeaux mixture, dichlofluanid, dithianon, dodine, dodine free base, ferbam, fluorofolpet, folpet, guazatine, guazatine acetate, iminoctadine, iminoctadine albesilate, iminoctadine triacetate, mancopper, mancozeb, maneb, metiram, metiram zinc, propineb, sulphur and sulphur preparations including calcium polysulphide, thiram, tolylfluanid, zineb, ziram;
- a compound selected in the following list amibromdole, benthiazole, bethoxazin, capsimycin, carvone, chinomethionat, chloropicrin, cufraneb, cyfiufenamid, cymoxanil, dazomet, debacarb, diclomezine, dichlorophen, dicloran, difenzoquat, difenzoquat methylsulphate, diphenylamine, ethaboxam, ferimzone, flumetover, flusulfamide, fosetyl-aluminium, fosetyl-calcium, fosetyl-sodium, fluopicolide, fluoroimide, hexachlorobenzene, 8-hydroxyquinoline sulfate, irumamycin, methasulphocarb, metrafenone, methyl isothiocyanate, mildiomycin, natamycin, nickel dimethyldithio
- composition according to the invention comprising a mixture of a compound of formula (I) with a bactericide compound may also be particularly advantageous.
- suitable bactericide mixing partners may be selected in the following list : bronopol, dichlorophen, nitrapyrin, nickel dimethyldithiocarbamate, kasugamycin, octhilinone, furancarboxylic acid, oxytetracycline, probenazole, streptomycin, tecloftalam, copper sulphate and other copper preparations.
- the fungicidal compositions of the present invention can be used to curatively or preventively control the phytopathogenic fungi of crops.
- a method for curatively or preventively controlling the phytopathogenic fungi of crops characterised in that a fungicidal composition as hereinbefore defined is applied to the seed, the plant and/or to the fruit of the plant or to the soil in which the plant is growing or in which it is desired to grow.
- composition as used against phytopathogenic fungi of crops comprises an effective and non-phytotoxic amount of an active material of general formula (I).
- an effective and non-phytotoxic amount means an amount of composition according to the invention which is sufficient to control or destroy the fungi present or liable to appear on the crops, and which does not entail any appreciable symptom of phytotoxicity for the said crops. Such an amount can vary within a wide range depending on the fungus to be controlled, the type of crop, the climatic conditions and the compounds included in the fungicidal composition according to the invention.
- the method of treatment according to the present invention is useful to treat propagation material such as tubers or rhizomes, but also seeds, seedlings or seedlings pricking out and plants or plants pricking out. This method of treatment can also be useful to treat roots.
- the method of treatment according to the present invention can also be useful to treat the overground parts of the plant such as trunks, stems or stalks, leaves, flowers and fruits of the concerned plant.
- cotton Among the plants that can be protected by the method according to the present invention, mention may be made of cotton; flax; vine; fruit or vegetable crops such as Rosaceae sp. (for instance pip fruit such as apples and pears, but also stone fruit such as apricots, almonds and peaches), Ribesioidae sp., Juglandaceae sp., Betulaceae sp., Anacardiaceae sp., Fagaceae sp., Moraceae sp., Oleaceae sp., Actinidaceae sp., Lauraceae sp., Musaceae sp.
- Rosaceae sp. for instance pip fruit such as apples and pears, but also stone fruit such as apricots, almonds and peaches
- Rosaceae sp. for instance pip fruit such as apples and pears, but also stone fruit such as apricots, almonds and peaches
- Rubiaceae sp. for instance banana trees and plantins
- Rubiaceae sp. Theaceae sp., Sterculiceae sp., Rutaceae sp. (for instance lemons, oranges and grapefruit); Solanaceae sp. (for instance tomatoes), Liliaceae sp., Asteraceae sp. (for instance lettuces), Umbelliferae sp., Cruciferae sp., Chenopodiaceae sp., Cucurbitaceae sp., Papilionaceae sp. (for instance peas), Rosaceae sp. (for instance strawberries); major crops such as Graminae sp.
- Asteraceae sp. for instance sunflower
- Cruciferae sp. for instance colza
- Fabacae sp. for instance peanuts
- Papilionaceae sp. for instance soybean
- Solanaceae sp. for instance potatoes
- Chenopodiaceae sp. for instance beetroots
- horticultural and forest crops as well as genetically modified homologues of these crops.
- Powdery mildew diseases such as :
- Blumeria diseases caused for example by Blumeria graminis
- Podosphaera diseases caused for example by Podosphaera leucotricha
- Sphaerotheca diseases caused for example by Sphaerotheca fuliginea
- Uncinula diseases caused for example by Uncinula necator
- Rust diseases such as :
- Gymnosporangium diseases caused for example by Gymnosporangium sabinae
- Hemileia diseases caused for example by Hemileia vastatrix
- Phakopsora diseases caused for example by Phakopsora pachyrhizi or Phakopsora meibomiae
- Puccinia diseases caused for example by Puccinia recondita
- Uromyces diseases caused for example by Uromyces appendiculatus
- Oomycete diseases such as :
- Bremia diseases caused for example by Bremia lactucae
- Peronospora diseases caused for example by Peronospora pisi or P. brassicae
- Phytophthora diseases caused for example by Phytophthora infestans
- Plasmopara diseases caused for example by Plasmopara viticola
- Pseudoperonospora diseases caused for example by Pseudoperonospora humuli or Pseudoperonospora cubensis
- Pythium diseases caused for example by Pythium ultimum
- Leafspot, leaf blotch and leaf blight diseases such as :
- Alternaria diseases caused for example by Alternaria solani; Cercospora diseases, caused for example by Cercospora beticola; Cladiosporum diseases, caused for example by Cladiosporium cucumerinum; Cochliobolus diseases, caused for example by Cochliobolus sativus;
- Colletotrichum diseases caused for example by Cottetotrichum lindemuthan ium ;
- Cycloconium diseases caused for example by Cycloconium oleaginum
- Diaporthe diseases caused for example by Diaporthe citri
- Elsinoe diseases caused for example by Elsinoe fawcettii
- Gloeosporium diseases caused for example by Gloeosporium laeticolor
- Glomerella diseases caused for example by Glomerella cingulata
- Guignardia diseases caused for example by Guignardia bidwelli;
- Leptosphaeria diseases caused for example by Leptosphaeria maculans; Leptosphaeria nodorum; Magnaporthe diseases, caused for example by Magnaporthe grisea;
- Mycosphaerella diseases caused for example by Mycosphaerella graminicola; Mycosphaerella arachidicola; Mycosphaerella ⁇ jiens is;
- Phaeosphaeria diseases caused for example by Phaeosphaeria nodorum
- Pyrenophora diseases caused for example by Pyrenophora teres
- Ramularia diseases caused for example by Ramularia collo-cygni
- Rhynchosporium diseases caused for example by Rhynchosporium secalis
- Septoria diseases caused for example by Septoria apii or Septoria lycopercisi;
- Typhula diseases caused for example by Typhula incarnata
- Venturia diseases caused for example by Venturia inaequalis
- Root and stem diseases such as :
- Corticium diseases caused for example by Corticium graminearum
- Fusarium diseases caused for example by Fusarium oxysporum
- Gaeumannomyces diseases caused for example by Gaeumannomyces graminis
- Rhizoctonia diseases caused for example by Rhizoctonia solani
- Tapesia diseases caused for example by Tapesia acuformis
- Thielaviopsis diseases caused for example by Thielaviopsis basicola; Ear and panicle diseases such as :
- Alternaria diseases caused for example by Alternaria spp.; Aspergillus diseases, caused for example by Aspergillus flavus;
- Cladosporium diseases caused for example by Cladosporium spp.;
- Claviceps diseases caused for example by Claviceps purpurea
- Fusarium diseases caused for example by Fusarium culmorum
- Gibberella diseases caused for example by Gibberella zeae
- Monographella diseases caused for example by Monographella nivalis
- Smut and bunt diseases such as :
- Sphacelotheca diseases caused for example by Sphacelotheca reiliana
- Tilletia diseases caused for example by Tilletia caries
- Urocystis diseases caused for example by Urocystis occulta
- Ustilago diseases caused for example by Ustilago nuda
- Fruit rot and mould diseases such as : Aspergillus diseases, caused for example by Aspergillus flavus;
- Botrytis diseases caused for example by Botrytis cinerea
- Penicillium diseases caused for example by Penicillium expansum
- Sclerotinia diseases caused for example by Sclerotinia sclerotiorum
- Verticilium diseases caused for example by Verticilium alboatrum
- Seed and soilborne decay, mould, wilt, rot and damping-off diseases Seed and soilborne decay, mould, wilt, rot and damping-off diseases :
- Fusarium diseases caused for example by Fusarium culmorum
- Phytophthora diseases caused for example by Phytophthora cactorum
- Pythium diseases caused for example by Pythium ultimum
- Rhizoctonia diseases caused for example by Rhizoctonia solani
- Sclerotium diseases caused for example by Sclerotium rolfsii;
- Microdochium diseases caused for example by Microdochium nivale; Canker, broom and dieback diseases such as :
- Nectria diseases caused for example by Nectria galligena; Blight diseases such as :
- Monilinia diseases caused for example by Monilinia laxa;
- Leaf blister or leaf curl diseases such as :
- Taphrina diseases caused for example by Taphrina deformans
- Decline diseases of wooden plants such as : Esca diseases, caused for example by Phaemoniella clamydospora;
- Botrytis diseases caused for example by Botrytis cinerea; Diseases of tubers such as :
- Rhizoctonia diseases caused for example by Rhizoctonia solani.
- the fungicide composition according to the present invention may also be used against fungal diseases liable to grow on or inside timber.
- the term "timber" means all types of species of wood, and all types of working of this wood intended for construction, for example solid wood, high-density wood, laminated wood, and plywood.
- the method for treating timber according to the invention mainly consists in contacting one or more compounds of the present invention, or a composition according to the invention; this includes for example direct application, spraying, dipping, injection or any other suitable means.
- the dose of active material usually applied in the treatment according to the present invention is generally and advantageously between 10 and 800 g/ha, preferably between 50 and 300 g/ha for applications in foliar treatment.
- the dose of active substance applied is generally and advantageously between 2 and 200 g per 100 kg of seed, preferably between 3 and 150 g per 100 kg of seed in the case of seed treatment. It is clearly understood that the doses indicated above are given as illustrative examples of the invention. A person skilled in the art will know how to adapt the application doses according to the nature of the crop to be treated.
- the fungicidal composition according to the present invention may also be used in the treatment of genetically modified organisms with the compounds according to the invention or the agrochemical compositions according to the invention.
- Genetically modified plants are plants into whose genome a heterologous gene encoding a protein of interest has been stably integrated.
- the expression "heterologous gene encoding a protein of interest” essentially means genes which give the transformed plant new agronomic properties, or genes for improving the agronomic quality of the transformed plant.
- compositions according to the present invention may also be used for the preparation of composition useful to curatively or preventively treat human and animal fungal diseases such as, for example, mycoses, dermatoses, trichophyton diseases and candidiases or diseases caused by Aspergillus spp., for example Aspergillus fumigatus.
- fungal diseases such as, for example, mycoses, dermatoses, trichophyton diseases and candidiases or diseases caused by Aspergillus spp., for example Aspergillus fumigatus.
- Example A in vivo test on Alternaria brassicae (Leaf spot of crucifers)
- the active ingredient tested is prepared by homogenisation in DMSO (5 % of the final volume), acetone (10% of the final volume) and tween 80 10% (5 ⁇ l/mg active ingredient).
- Radish plants (Pernot variety) in starter cups, sown on a 50/50 peat soil-pozzolana substrate and grown at 18-20 0 C, are treated at the cotyledon stage by spraying with the aqueous suspension described above.
- Plants, used as controls, are treated with an aqueous solution not containing the active material.
- the plants are contaminated by spraying them with an aqueous suspension of Alternaria brassicae spores (40,000 spores per cm 3 ).
- the spores are collected from a 12 to 13 days-old culture.
- the contaminated radish plants are incubated for 6-7 days at about 18°C, under a humid atmosphere.
- Example B in vivo test on Botrvtis cinerea (cucumber Grey mould)
- the active ingredient tested is prepared by homogenisation in DMSO (5 % of the final volume), acetone (10% of the final volume) and tween 80 10% (5 ⁇ l/mg active ingredient).
- Cucumber plants (Marketer variety) in starter cups, sown on a 50/50 peat soil-pozzolana substrate and grown at 18- 20 0 C, are treated at the cotyledon ZI l stage by spraying with the aqueous suspension described above. Plants, used as controls, are treated with an aqueous solution not containing the active material. After 24 hours, the plants are contaminated by depositing drops of an aqueous suspension o ⁇ Botrytis cinerea spores (150,000 spores per ml) on upper surface of the leaves. The spores are collected from a 15-day-old culture and are suspended in a nutrient solution composed of :
- the contaminated cucumber plants are settled for 5/7 days in a climatic room at 15-11 0 C (day/night) and at 80% relative humidity. Grading is carried out 5/7 days after the contamination, in comparison with the control plants. Under these conditions, good (at least 70%) or total protection is observed :
- Example C in vivo test on Pyrenophora teres (Barley Net blotch)
- the active ingredient tested is prepared by homogenisation in DMSO (5 % of the final volume), acetone (10% of the final volume) and tween 80 10% (5 ⁇ l/mg active ingredient).
- Barley plants (Express variety) in starter cups, sown on a 50/50 peat soil-pozzolana substrate and grown at 12°C, are treated at the 1-leaf stage (10 cm tall) by spraying with the aqueous suspension described above. Plants, used as controls, are treated with an aqueous solution not containing the active material. After 24 hours, the plants are contaminated by spraying them with an aqueous suspension of Pyrenophora teres spores (12,000 spores per ml).
- the spores are collected from a 12-day-old culture .
- the contaminated barley plants are incubated for 24 hours at about 20 0 C and at 100% relative humidity, and then for 12 days at 80% relative humidity. Grading is carried out 12 days after the contamination, in comparison with the control plants. Under these conditions, good (at least 70%) or total protection is observed :
- N-sec-butyl-2,5-dimethyl-3-furamide disclosed in GB patent application GB 1387652 did not show any activity against Pyrenophora teres.
- Example D in vivo test on Svhaerotheca fulisinea (powdery mildew)
- the active ingredient tested is prepared by homogenisation in DMSO (5 % of the final volume), acetone (10% of the final volume) and tween 80 10% (5 ⁇ l/mg active ingredient).
- Gherkin plants (Vert petit de Paris variety) in starter cups, sown on a 50/50 peat soil-pozzolana substrate and grown at 20°C/23°C, are treated at cotyledon ZI l stage by spraying with the aqueous suspension described above. Plants, used as controls, are treated with an aqueous solution not containing the active material. After 24 hours, the plants are contaminated by spraying them with an aqueous suspension of Sphaerotheca fuliginea spores (100 000 spores per ml). The spores are collected from a contaminated plants . The contaminated gerkhin plants are incubated at about 20°C/25°C and at 60/70% relative humidity. Grading (% of efficacy) is carried out 21 days after the contamination, in comparison with the control plants. Under these conditions, good (at least 70%) or total protection is observed :
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Plant Pathology (AREA)
- Pest Control & Pesticides (AREA)
- Health & Medical Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
- Furan Compounds (AREA)
- Pyrrole Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Heterocyclic Compounds Containing Sulfur Atoms (AREA)
- Thiazole And Isothizaole Compounds (AREA)
Abstract
A compound of general formula (I). A process for preparing this compound. A compound of general formula (II) A fungicide composition comprising a compound of general formula (I). A method for treating plants by applying a compound of general formula (I) or a composition comprising it.
Description
New N-alkyl-heterocvclyl carboxamide derivatives
The present invention relates to novel N-alkyl-heterocyclyl carboxamide derivatives, their process of preparation, their use as fungicides, particularly in the form of fungicidal compositions, and methods for the control of phytopathogenic fungi of plants using these compounds or their compositions.
International patent application WO 96/38419 discloses pyrazole carboxamide derivatives of general formula encompassing the compounds according to the present invention, and their use as fungicide. However, compounds according to the present invention are not disclosed in that patent application.
International patent application WO 96/29871 discloses thiadiazole carboxamide derivatives of general formula encompassing the compounds according to the present invention, and their use as fungicide. However, compounds according to the present invention are not disclosed in that patent application.
Great Britain patent application GB 1387652 discloses furan carboxamide derivatives of general formula encompassing the compounds according to the present invention, and their use as fungicide. However, compounds according to the present invention are not disclosed in that patent application.
Japanese patent application JP 9176124 discloses pyrazolin carboxamide derivatives of general formula encompassing the compounds according to the present invention, and their use as fungicide. However, compounds according to the present invention are not disclosed in that patent application. US patent application US 5,240,951 discloses isothiazole carboxamide derivatives of general formula encompassing the compounds according to the present invention, and their use as fungicide. However, compounds according to the present invention are not disclosed in that patent application.
It is always of high-interest in the field of agrochemicals to use pesticidal compounds more active than the compounds already known by the man ordinary skilled in the art whereby less compound can be used whilst retaining equivalent efficacy.
Furthermore, the provision of new pesticidal compounds with a higher efficacy strongly reduces the risk of appearance of resistant strains in the fungi to be treated.
We have now found a new family of compounds which show enhanced fungicidal activity over the general known family of such compounds.
Accordingly, the present invention relates to a N-alkyl-heterocyclyl carboxamide derivative of general formula (I)
- Het represents 5-membered non-fused heterocycle with one, two or three heteroatoms which may be the same or different, Het being linked by a carbon atom and being at least substituted in ortho position;
- n is 2, 3 or 4;
- R1 is chosen as being a hydrogen atom, a Ci-C6-alkyl group or a C3-C7- cycloalkyl;
- R is chosen as being a Ci-C6-alkyl group; - R3 is chosen as being a hydrogen atom or a Ci-Cδ-alkyl group;
- each X is chosen, independently of the others, as being a hydrogen atom or a Ci-Ce-alkyl group;
- W represents a carbon atom or a silicon atom;
- R4, R5 and R6 are chosen, independently of each other, as being a hydrogen atom, a halogen atom, or a Ci-C6-alkyl group, at least two of the substituents R4, R5 and R6 being different from a hydrogen atom; or two of the substituents R4, R5 and R6 may together form a 3-, 4-, 5- or 6- membered non aromatic cycle optionally substituted with a Ci-C6-alkyl group; as well as its salts, N-oxydes, metallic complexes, metalloidic complexes and optically active isomers with the exception of :
5-(dimethylamino)-N-(l,5-dimethylhexyl)-4-methyl-4H-l,2,4-triazole-3- carboxamide;
N-(l,5-dimethylhexyl)-l-[4-[[(l,5-dimethylhexyl)amino]sulfonyl]phenyl]-4-[[4- [[(l,5-dimethylhexyl)amino]sulfonyl]phenyl]azo]-5-hydroxy-lH-pyrazole-3- carboxamide;
5 -amino-N-( 1 ,5 -dimethylhexyl)-3 - {5 - [3 -(trifluoromethyl)phenyl] -2- furyl} isoxazole-4-carboxamide;
- 5-amino-3-(5-bromothien-2-yl)-N-(l,5-dimethylhexyl)isoxazole-4-carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(5-methylthien-2-yl)isoxazole-4-carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(3-methylthien-2-yl)isoxazole-4-carboxamide;
5-amino-3-[5-(3-chlorophenyl)-2-furyl]-N-(l,5-dimethylhexyl)isoxazole-4- carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(5-methyl-2-furyl)isoxazole-4-carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(2-furyl)isoxazole-4-carboxamide;
- 5-amino-3-(l-benzofuran-3-yl)-N-(l,5-dimethylhexyl)isoxazole-4-carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(5-ethylthien-2-yl)isoxazole-4-carboxamide; - 5-amino-3-(5-chloro-2-furyl)-N-(l ,5-dimethylhexyl)isoxazole-4-carboxamide;
5-amino-N-(l,5-dimethylhexyl)-3-(6-methoxypyridin-3-yl)isoxazole-4- carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-pyridin-3-ylisoxazole-4-carboxamide;
- 5-(4-chlorophenyl)-l-(2,4-dichlorophenyl)-N-(l,4-dimethylpentyl)- 4-methyl-lH-pyrazole-3 -carboxamide; and
- 5-(4-chlorophenyl)-l-(2,4-dichlorophenyl)-N-(l,5-dimethylhexyl)- 4-methyl-lH-pyrazole-3 -carboxamide.
In the context of the present invention : - halogen means fluorine, bromine, chlorine or iodine.
-carboxy means -C(=O)OH ; carbonyl means -C(=O)- ; carbamoyl means - C(=O)NH2 ; N-hydroxycarbamoyl means -C(=O)NHOH ;
- an alkyl group, an alkenyl group, and an alkynyl group as well as moieties containing these terms, can be linear or branched. In the context of the present invention, it has also to be understood that in the case of di-substituted amino and of di-substituted carbamoyl radicals, the two substituents may form together with the nitrogen atom bearing them a saturated heterocyclic ring containing 3 to 7 atoms.
Any of the compound of the present invention can exist in one or more optical or chiral isomer forms depending on the number of asymmetric centres in the compound. The invention thus relates equally to all the optical isomers and to their racemic or scalemic mixtures (the term "scalemic" denotes a mixture of enantiomers in different proportions), and to the mixtures of all the possible stereoisomers, in all proportions. The diastereoisomers and/or the optical isomers can be separated according to the methods which are known per se by the man ordinary skilled in the art.
Any of the compound of the present invention can also exist in one or more geometric isomer forms depending on the number of double bonds in the compound.
The invention thus relates equally to all geometric isomers and to all possible mixtures, in all proportions. The geometric isomers can be separated according to general methods, which are known per se by the man ordinary skilled in the art.
According to the present invention, "Het" of the compound of general formula (I) is a 5-membered non-fused heterocycle with one, two or three heteroatoms which may be the same or different, Het being linked by a carbon atom and being at least substituted in ortho position. Preferably, the present invention relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I) in which the different characteristics may be chosen alone or in combination as being :
- as regards Het moiety, Het is chosen as being 2-furan, 3-furan, 4,5-dihydro-3-furan, 2-thiophene, 3-thiophene, 2-pyrrole, 3-pyrrole, 5-oxazole, 4-oxazole, 5-thiazole, A- thiazole, 5-pyrazole, 4-pyrazole, 3-pyrazole, 3-isoxazole, 4-isoxazole, 5-isoxazole, 3- isothiazole, 4-1,2,3-triazole, 4-thiadiazole or 5-thiadiazole;
- as regards the substituents of the "Het" moiety, each substituent is chosen, independently of the others, as being a hydrogen atom, a halogen atom, an amino group, a cyano group, a Ci-C4-alkylamino, a di-(Ci-C4-alkyl)amino, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms, a nitro group, a Ci-C4-alkyloxy, a Ci-C4-alkylthio, a C1-C4- alkylsulphonyl, a phenyl optionally substituted by a halogen atom or a Ci-C4-alkyl or a pyridyl optionally substituted by a halogen atom or a Ci-C4-alkyl, a di(Ci-C4- alkyl)aminosulphonyl, a Ci-C6-alkylcarbonyl, a phenylsulphonyl optionally substituted by a halogen atom or a Ci-C4-alkyl, or a benzoyl optionally substituted by a halogen atom or a Ci-C4-alkyl, a carbamoyl group or an a Ci-Cs- alkylcarbonylamino, a C2-C6-alkenyl, a C3-C6-cycloalkyl, a Ci-C4-alkylthio-Ci-C4- alkyl, a Ci-C4-halogenoalkylthio-Ci-C4-alkyl having 1 to 5 halogen atoms, a C1-C4- alkoxy-Ci-C4-alkyl or a Ci-C4-halogenoalkoxy-Ci-C4-alkyl having 1 to 5 halogen atoms;
- as regards the substituent in 2-position of the "Het" moiety, the substituent in ortho position of the "Het" moiety is chosen as being a halogen atom, a Ci-C4-alkyl, a Ci- C4-halogenoalkyl having 1 to 5 halogen atoms, a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms or a Ci-C4-alkyloxy;
Specific examples of Het moiety include :
* Het represents a heterocycle of the general formula (Het-1)
- R7 and R8 may be the same or different and may be a hydrogen atom, a halogen atom, an amino group, a nitro group, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms; and
- R9 may be a halogen atom, a nitro group, a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms.
* Het represents a heterocycle of the general formula (Het-2)
in which : - R10 may be a hydrogen atom, a halogen atom , a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms; and
- R11 and R12 may be the same or different and may be a hydrogen atom, a halogen atom, an amino group, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms; provided that the R10 and R12 are not both a hydrogen atom.
* Het represents a heterocycle of the general formula (Het-3)
(Het-3) in which :
- R13 may be a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms; and - R14 may be a hydrogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having
1 to 5 halogen atoms.
Het represents a heterocycle of the general formula (Het-4)
(Het-4) in which :
- R15 and R16 may be the same or different and may be a hydrogen atom, a halogen atom, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a
Ci-C4-alkylthio, a Ci-C4-alkylsulphonyl, a phenyl optionally substituted by a halogen atom or a Ci-C4-alkyl or a pyridyl otpionally substituted by a halogen atom or a Ci-
C4-alkyl; and
- R17 may be a halogen atom, a cyano group, a Ci-C4-alkyl, a C1-C4- halogenoalkyl having 1 to 5 halogen atoms or a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms.
Het represents a heterocycle of the general formula (Het-5)
(Het-5) in which :
- R , 18 and i π R19 may be the same or different and may be a hydrogen atom, a halogen atom, a Ci-C4-alkyl, a Ci-C4-alkyloxy or a Ci-C4-halogenoalkyl having 1 to
5 halogen atoms; and
- R20 may be a hydrogen atom, a halogen atom, a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms; provided that the R19 and R20 are not both a hydrogen atom.
* Het represents a heterocycle of the general formula (Het-6)
- R21 may be a hydrogen atom, a halogen atom, a cyano group, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms;
- R22 and R24 may be the same or different and may be a hydrogen atom, a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms; and
- R23 may be a hydrogen atom, a cyano group, a Ci-C4-alkyl, a C1-C4- halogenoalkyl having 1 to 5 halogen atoms, a Ci-C4-alkoxy-Ci-C4-alkyl, a hydroxy- Ci-C4-alkyl, a Ci-C4-alkylsulphonyl, a di(Ci-C4-alkyl)aminosulphonyl, a Ci-C6- alkylcarbonyl, a phenylsulphonyl optionally substituted by a halogen atom or a Ci- C4-alkyl, or a benzoyl optionally substituted by a halogen atom or a Ci-C4-alkyl; provided that the R21 and R24 are not both a hydrogen atom.
* Het represents a heterocycle of the general formula (Het-7)
- R25 may be a hydrogen atom, a cyano group, a Ci-C4-alkyl, a C1-C4- halogenoalkyl having 1 to 5 halogen atoms, a Ci-C4-alkoxy-Ci-C4-alkyl, a hydroxy- Ci-C4-alkyl, a Ci-C4-alkylsulphonyl, a di(Ci-C4-alkyl)aminosulphonyl, a Ci-C6- alkylcarbonyl, a phenylsulphonyl optionally substituted by a halogen atom or a Ci- C4-alkyl, or a benzoyl optionally substituted by a halogen atom or a Ci-C4-alkyl; and
- R26, R27 and R28 may be the same or different and may be a hydrogen atom, a halogen atom, a cyano group, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms or a Ci-C4-alkylcarbonyl; provided that R25 and R28 are not both a hydrogen atom.
* Het represents a heterocycle of the general formula (Het-8)
- R30 may be a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms.
* Het represents a heterocycle of the general formula (Het-9)
(Het-9) in which :
- R31 may be a hydrogen atom or a Ci-C4-alkyl; and
- R32 may be a halogen atom, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms or a phenyl optionally substituted by a halogen atom or a C1-C4- alkyl.
* Het represents a heterocycle of the general formula (Het- 10)
(Het- 10) in which :
- R33 may be a hydrogen atom, a halogen atom, an amino group, a cyano group, a Ci-C4-alkylamino, a di-(Ci-C4-alkyl)amino, a Ci-C4-alkyl, a C1-C4- halogenoalkyl having 1 to 5 halogen atoms or a phenyl optionally substituted by a halogen atom or a d-C^alkyl; and
- R34 may be a halogen atom, a Q-Gralkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms.
* Het represents a heterocycle of the general formula (Het-11)
(Het-11) in which :
- R35 may be a hydrogen atom, a halogen atom, an amino group, a cyano group, a Ci-C4-alkylamino, a di-(Ci-C4-alkyl)amino, a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms; and
-R36 may be a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms.
* Het represents a heterocycle of the general formula (Het-12)
- R37 may be a halogen atom, a cyano group, a nitro group, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a C3-C6-cycloalkyl, a C1-C4- alkoxy, a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms, a Ci-C4-alkylthio, a Ci-C4-halogenoalkylthio having 1 to 5 halogen atoms, an aminocarbonyl group or an aminocarbonyl-Ci-C4-alkyl;
- R38 may be a hydrogen atom, a halogen atom, a cyano group, a nitro group, a Ci-C4-alkyl, a Ci-C4-alkoxy or a Ci-C4-alkylthio; and
- R39 may be a hydrogen atom, a phenyl, a Ci-C4-alkyl, a C1-C4- halogenoalkyl having 1 to 5 halogen atoms, a hydroxy-Ci-C4-alkyl, a C2-C6-alkenyl, a C3-C6-cycloalkyl, a Ci-C4-alkylthio-Ci-C4-alkyl, a Ci-C4-halogenoalkylthio-Ci-C4- alkyl having 1 to 5 halogen atoms, a Ci-C4-alkoxy-Ci-C4-alkyl or a C1-C4- halogenoalkoxy-Ci-C4-alkyl having 1 to 5 halogen atoms.
* Het represents a heterocycle of the general formula (Het- 13)
- R40 may be a hydrogen atom, a halogen atom, a cyano group, a nitro group, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a C3-C6- cycloalkyl, a Ci-C4-alkoxy, a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms, a
Ci-C4-alkylthio, a Ci-C4-halogenoalkylthio having 1 to 5 halogen atoms, an aminocarbonyl or an aminocarbonyl-Ci-C4-alkyl;
- R41 may be a hydrogen atom, a halogen atom, a cyano group, a Ci-C4-alkyl, a Ci-C4-alkoxy, a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms or a C1-C4- alkylthio; and
- R42 may be a hydrogen atom, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a hydroxy-Ci-C4-alkyl, a C2-C6-alkenyl, a C3-C6-cycloalkyl, a Ci-C4-alkylthio-Ci-C4-alkyl, a Ci-C4-halogenoalkylthio-Ci-C4-alkyl having 1 to 5 halogen atoms, a Ci-C4-alkoxy-Ci-C4-alkyl, a Ci-C4-halogenoalkoxy-Ci-C4-alkyl having 1 to 5 halogen atoms or a phenyl optionally substituted by a halogen atom, a Ci-C4-alkyl, a Ci-C4-alkoxyalkyl or a nitro group; provided that the R40 and R41 are not both a hydrogen atom.
* Het represents a heterocycle of the general formula (Het-14)
-R43 may be a hydrogen atom, a halogen atom, a cyano group, a nitro group, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a C3-C6-cycloalkyl, a Ci-C4-alkoxy, a Ci-C4-halogenoalkoxy having 1 to 5 halogen atoms, a C1-C4- alkylthio, a Ci-C4-halogenoalkylthio having 1 to 5 halogen atoms, an aminocarbonyl, or an aminocarbonyl-Ci-C4-alkyl;
- R44 may be a hydrogen atom, a halogen atom, a cyano group, a Ci-C4-alkyl, a Ci-C4-alkoxy, a Ci-C4-alkylthio or a Ci-C4-halogenoalky having 1 to 5 halogen atoms;
- R45 may be a hydrogen atom, a phenyl, a benzyl, a Ci-C4-alkyl, a C1-C4- halogenoalkyl having 1 to 5 halogen atoms, a hydroxy-Ci-C4-alkyl, a C2-C6-alkenyl, a C3-C6-cycloalkyl, a Ci-C4-alkylthio-Ci-C4-alkyl, a Ci-C4-halogenoalkylthio-Ci-C4- alkyl having 1 to 5 halogen atoms, a Ci-C4-alkoxy-Ci-C4-alkyl, a C1-C4- halogenoalkoxy-Ci-C4-alkyl having 1 to 5 halogen atoms; provided that R44 and R45 are not both a hydrogen atom.
Het represents a heterocycle of the general formula (Het-15)
- R46 may be a hydrogen atom, a halogen atom, a C-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms; and - R47 may be a halogen atom, a C-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms.
Het represents a heterocycle of the general formula (Het- 16)
in which R48 and R49 may be the same or different and may be a hydrogen atom, a halogen atom, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a phenyl optionally substituted by a halogen atom or a Ci-C4-alkyl, or a heterocyclyl optionally substituted by a halogen atom or a Ci-C4-alkyl; provided that R48 and R498 are not both a hydrogen atom.
* Het represents a heterocycle of the general formula (Het- 17)
1 to 5 halogen atoms, and
- R > 51 may be a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having
1 to 5 halogen atoms.
Het represents a heterocycle of the general formula (Het-18)
in which R52 may uc a iicuυgcii ciLUiii, a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms.
* Het represents a heterocycle of the general formula (Het- 19)
in which :
- R53 may be a halogen atom, a Ci-C4-alkyl or a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms; and
- R54 may be a hydrogen atom, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, or a phenyl optionally substituted by a halogen atom or a C1-C4- alkyl.
* Het represents a heterocycle of the general formula (Het-20)
in which R55 may be a halogen atom, a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms.
* Het may represent a heterocycle of the general formula (Het-21)
in which R56 may be a halogen atom, a Ci-C4-alkyl or a C1-C4- halogenoalkyl having 1 to 5 halogen atoms.
According to the present invention, the nitrogen atom of the carboxamide moiety of the compound of formula (I) is substituted by R1, R1 being a hydrogen atom or a C3-C7-cycloalkyl.. Preferably, the C3-C7-CyC loalkyl is cyclopropyl.
According to the present invention, the first carbon atom of the alkyl chain of the compound of formula (I) is substituted by R2, R2 being as defined above.
Preferably, the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I) in which R2 may be chosen as being a Ci-C3 alkyl.
More preferably, R is a methyl group.
According to the present invention, the alkyl chain of the compound of formula (I) further comprises n carbon atoms, each of which being substituted by R3, R3 being as defined above. Preferably, the present invention also relates to N-alkyl- heterocyclyl carboxamide derivative of general formula (I) in which the different characteristics may be chosen alone or in combination as being :
- as regards n, n is 3; and
- as regards R3, R3 is a methyl group.
According to the present invention, the last carbon atom of the alkyl chain of the compound of formula (I) is substituted by R4, R5 and R6, R4, R5 and R6 being as defined above. Preferably, the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I) in which R4, R5 and R6 may be chosen, independently of each other as being a hydrogen atom or a methyl group.
According to the present invention, the alkyl chain of the compound of formula (I) contains from 2 to 4 carbon atoms, each of which is substituted by a substituent X which may the same or different. Preferably, the present invention relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I) in which X is chosen as being a hydrogen atom or a methyl group.
Preferably, the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I-a)
in which :
- Het, R1, R2, R3, R4, R5 and R6 are as defined above; and
- X1 and X2 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
Preferably, the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I-b)
- Het, R1, R2, R3, R4, R5 and R6 are as defined above; and
- X1, X2 and X3 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
Preferably, the present invention also relates to N-alkyl-heterocyclyl carboxamide derivative of general formula (I-c)
- Het, R1, R2, R3, R4, R5 and R6 are as defined above; and
- X1, X2, X3 and X4 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
The present invention also relates to a process for the preparation of the compound of general formula (I). Thus, according to a further aspect of the present invention there is provided a process for the preparation of compound of general
formula (I) as defined above, which comprises reacting an alkyl-amine derivative of general formula (II) or one of its salt
in which R1, R2, R3, X, n, W, R4, R5 and R6 are as defined above; with a carboxylic acid derivative of the general formula (III)
-Het is as defined above; and - L1 is a leaving group chosen as being a halogen atom, a hydroxyl group, -
OR54, -OCOR54, R54 being a Ci-C6 alkyl, a Ci-C6 haloalkyl, a benzyl, 4- methoxybenzyl, pentafluorophenyl or a group of formula j? ;
O Het in the presence of a catalyst and, if L1 is a hydroxyl group, in the presence of a condensing agent.
The process according to the present invention is conducted in the presence of a catalyst. Suitable catalyst may be chosen as being 4-dimethyl-aminopyridine, 1- hydroxy-benzotriazole or dimethylformamide. In case L1 is a hydroxy group, the process according to the present invention is conducted in the presence of condensing agent. Suitable condensing agent may be chosen as being acid halide former, such as phosgene, phosphorous tribromide, phosphorous trichloride, phosphorous pentachloride, phosphorous trichloride oxide or thionyl chloride; anhydride former, such as ethyl chloroformate, methyl chloroformate, isopropyl chloroformate, isobutyl chloroformate or methanesulfonyl- chloride; carbodiimides, such as N,N'-dicyclohexylcarbodiimide (DCC) or other customary condensing agents, such as phosphorous pentoxide, polyphosphoric acid, N,N'-carbonyl-diimidazole, 2-ethoxy-N-ethoxycarbonyl- 1 ,2-dihydroquinoline
(EEDQ), triphenylphosphine/tetrachloromethane, 4-(4,6-dimethoxy[l .3.5]triazin-2-
yl)-4-methylmorpholinium chloride hydrate or bromo-tripyrrolidino-phosphonium- hexafluorophosphate.
When R1 is a hydrogen atom, the above mentioned process for the preparation of compound of general formula (I) may optionally be completed by a further step according to the following reaction scheme :
(Ib) (VIII) (Ia)
in which : - R2, R3, X, n, W, R4, R5, R6 and Het are as defined above;
- L2 is a leaving group chosen as being a halogen atom, a 4- methyl phenylsulfonyloxy or a methylsulfonyloxy; and
- R1 a is a Ci-C6-alkyl group or a C3-Cy-cycloalkyl; comprising the reaction of a compound of general formula (Ib) with a compound of general formula (VIII) to provide a compound of general formula (Ia).
Depending on the definition of R1, R2, R3, X, n, R4, R5 and R6, amine derivatives of general formula (II) may be prepared by different processes. One example (A) of such a process may be when :
- R1, R2, X, n, W, R4, R5 and R6 are as defined above; and
- R3 is a hydrogen atom; then, the amine derivative of general formula (II) may be prepared according to a process which comprises :
- a first step according to reaction scheme A-I :
Scheme A-I
(IV) (V) in which R1, R2, X, n, W, R4, R5 and R6 are as defined above;
comprising the reaction of a compound of general formula (IV) with an amine of formula R1-NH2 to provide an imine derivative of general formula (V); - a second step according to scheme A-2 :
Scheme A-2
(V) (Ha) in which R1, R2, X, n, W, R4, R5 and R6 are as defined above; comprising the reduction of an imine derivative of general formula (V) by hydrogenation or by an hydride donor, in the same or a different pot to provide an amine derivative of general formula (Ha) or one of its salt.
An other example (B) of such a process may be when :
- R2, R3, X, n, W, R4, R5, R6 are as defined above;
- R1 is a hydrogen atom then, the amine derivative of general formula (II) may be prepared according to a process which comprises :
- a first step according to reaction scheme B-I :
Scheme B-I
- R2, R3, X, n, W, R4, R5 and R6 are as defined above; and
- U is a leaving group chosen as being a halogen atom, a Ci-C6 alkylsulfonate or a Ci-C6 haloalkylsulfonate; comprising the nucleophilic substitution of a compound of general formula (VI) by a phtalimide salt to produce a compound of general formula (VII); - a second step according to reaction scheme B-2 :
Scheme B-2
in which R2, R3, X, n, W, R4, R5, R6 are as defined above; comprising the de-protection of a compound of general formula (VII) by reacting it with hydrazine hydrate or a hydrazine salt to provide an amine derivative of general formula (lib) or one of its salt;
The compound of general formula (II) used as an intermediate for the preparation of compound of general formula (I) is novel. Therefore, the present invention also relates to novel intermediate compound useful for the preparation of compound of general formula (I). Thus, according to the present invention, there is provided a compound of general formula (II)
The present invention also relates to a fungicidal composition comprising an effective amount of an active material of general formula (I). Thus, according to the present invention, there is provided a fungicidal composition comprising, as an active ingredient, an effective amount of a compound of general formula (I) as defined above and an agriculturally acceptable support, carrier or filler. In the present specification, the term "support" denotes a natural or synthetic, organic or inorganic material with which the active material is combined to make it easier to apply, notably to the parts of the plant. This support is thus generally inert and should be agriculturally acceptable. The support may be a solid or a liquid. Examples of suitable supports include clays, natural or synthetic silicates, silica, resins, waxes, solid fertilisers, water, alcohols, in particular butanol, organic solvents, mineral and plant oils and derivatives thereof. Mixtures of such supports may also be used.
The composition may also comprise additional components. In particular, the composition may further comprise a surfactant. The surfactant can be an emulsifier, a dispersing agent or a wetting agent of ionic or non-ionic type or a mixture of such surfactants. Mention may be made, for example, of polyacrylic acid salts, lignosulphonic acid salts, phenolsulphonic or naphthalenesulphonic acid salts, polycondensates of ethylene oxide with fatty alcohols or with fatty acids or with fatty amines, substituted phenols (in particular alkylphenols or arylphenols), salts of sulphosuccinic acid esters, taurine derivatives (in particular alkyl taurates), phosphoric esters of polyoxyethylated alcohols or phenols, fatty acid esters of polyols, and derivatives of the above compounds containing sulphate, sulphonate and phosphate functions. The presence of at least one surfactant is generally essential when the active material and/or the inert support are water- insoluble and when the vector agent for the application is water. Preferably, surfactant content may be comprised between 5% and 40% by weight of the composition. Optionally, additional components may also be included, e.g. protective colloids, adhesives, thickeners, thixotropic agents, penetration agents, stabilisers, sequestering agents. More generally, the active materials can be combined with any solid or liquid additive, which complies with the usual formulation techniques.
In general, the composition according to the invention may contain from 0.05 to 99% (by weight) of active material, preferably 10 to 70% by weight.
Compositions according to the present invention can be used in various forms such as aerosol dispenser, capsule suspension, cold fogging concentrate, dustable powder, emulsifiable concentrate, emulsion oil in water, emulsion water in oil, encapsulated granule, fine granule, fiowable concentrate for seed treatment, gas (under pressure),gas generating product, granule, hot fogging concentrate, macrogranule, microgranule, oil dispersible powder, oil miscible fiowable concentrate, oil miscible liquid, paste, plant rodlet, powder for dry seed treatment, seed coated with a pesticide, soluble concentrate, soluble powder, solution for seed treatment, suspension concentrate (fiowable concentrate), ultra low volume (ulv) liquid, ultra low volume (ulv) suspension, water dispersible granules or tablets, water dispersible powder for slurry treatment, water soluble granules or tablets, water soluble powder for seed treatment and wettable powder.
These compositions include not only compositions which are ready to be applied to the plant or seed to be treated by means of a suitable device, such as a spraying or dusting device, but also concentrated commercial compositions which must be diluted before application to the crop.
The compounds of the invention can also be mixed with one or more insecticides, fungicides, bactericides, attractant acaricides or pheromones or other compounds with biological activity. The mixtures thus obtained have a broadened spectrum of activity. The mixtures with other fungicides are particularly advantageous.
Examples of suitable fungicide mixing partners may be selected in the following lists :
1) a compound capable to inhibit the nucleic acid synthesis like benalaxyl, benalaxyl-M, bupirimate, chiralaxyl, clozylacon, dimethirimol, ethirimol, furalaxyl, hymexazol, metalaxyl-M, ofurace, oxadixyl, oxolinic acid ; 2) a compound capable to inhibit the mitosis and cell division like benomyl, carbendazim, diethofencarb, fuberidazole, pencycuron, thiabendazole thiophanate- methyl, zoxamide;
3) a compound capable to inhibit the respiration for example as Cl-respiration inhibitor like diflumetorim; as Cll-respiration inhibitor like boscalid, carboxin, fenfuram, flutolanil, furametpyr, mepronil, oxycarboxine, penthiopyrad, thifiuzamide; as CHI-respiration inhibitor like azoxystrobin, cyazofamid, dimoxystrobin, enestrobin, famoxadone, fenamidone, fluoxastrobin, kresoxim-methyl, metominostrobin, orysastrobin, pyraclostrobin, picoxystrobin, trifloxystrobin; 4) a compound capable of to act as an uncoupler like dinocap, fluazinam;
5) a compound capable to inhibit ATP production like fentin acetate, fentin chloride, fentin hydroxide, silthiofam;
6) a compound capable to inhibit AA and protein biosynthesis like andoprim, blasticidin-S, cyprodinil, kasugamycin, kasugamycin hydrochloride hydrate, mepanipyrim, pyrimethanil;
7) a compound capable to inhibit the signal transduction like fenpiclonil, fludioxonil, quinoxyfen;
8) a compound capable to inhibit lipid and membrane synthesis like chlozolinate, iprodione, procymidone, vinclozolin, pyrazophos, edifenphos, iprobenfos (IBP), isoprothiolane, tolclofos-methyl, biphenyl, iodocarb, propamocarb, propamocarb-hydrochloride;
9) a compound capable to inhibit ergosterol biosynthesis like fenhexamid, azaconazole, bitertanol, bromuconazole, cyproconazole, diclobutrazole, difenoconazole, diniconazole, diniconazole-M, epoxiconazole, etaconazole, fenbuconazole, fluquinconazole, fiusilazole, fiutriafol, furconazole, furconazole-cis, hexaconazole, imibenconazole, ipconazole, metconazole, myclobutanil,
paclobutrazol, penconazole, propiconazole, prothioconazole, simeconazole, tebuconazole, tetraconazole, triadimefon, triadimenol, triticonazole, uniconazole, voriconazole, imazalil, imazalil sulfate, oxpoconazole, fenarimol, flurprimidol, nuarimol, pyrifenox, triforine, pefurazoate, prochloraz, trifiumizole, viniconazole, aldimorph, dodemorph, dodemorph acetate, fenpropimorph, tridemorph, fenpropidin, spiroxamine, naftifine, pyributicarb, terbinafine;
10) a compound capable to inhibit cell wall synthesis like benthiavalicarb, bialaphos, dimethomorph, flumorph, iprovalicarb, polyoxins, polyoxorim, validamycin A; 11) a compound capable to inhibit melanine biosynthesis like carpropamid, diclocymet, fenoxanil, phtalide, pyroquilon, tricyclazole;
12) a compound capable to induce a host defence like acibenzolar-S-methyl, probenazole, tiadinil;
13) a compound capable to have a multisite action like captafol, captan, chlorothalonil, copper preparations such as copper hydroxide, copper naphthenate, copper oxychloride, copper sulphate, copper oxide, oxine-copper and Bordeaux mixture, dichlofluanid, dithianon, dodine, dodine free base, ferbam, fluorofolpet, folpet, guazatine, guazatine acetate, iminoctadine, iminoctadine albesilate, iminoctadine triacetate, mancopper, mancozeb, maneb, metiram, metiram zinc, propineb, sulphur and sulphur preparations including calcium polysulphide, thiram, tolylfluanid, zineb, ziram;
14) a compound selected in the following list: amibromdole, benthiazole, bethoxazin, capsimycin, carvone, chinomethionat, chloropicrin, cufraneb, cyfiufenamid, cymoxanil, dazomet, debacarb, diclomezine, dichlorophen, dicloran, difenzoquat, difenzoquat methylsulphate, diphenylamine, ethaboxam, ferimzone, flumetover, flusulfamide, fosetyl-aluminium, fosetyl-calcium, fosetyl-sodium, fluopicolide, fluoroimide, hexachlorobenzene, 8-hydroxyquinoline sulfate, irumamycin, methasulphocarb, metrafenone, methyl isothiocyanate, mildiomycin, natamycin, nickel dimethyldithiocarbamate, nitrothal-isopropyl,octhilinone, oxamocarb, oxyfenthiin, pentachlorophenol and salts, 2-phenylphenol and salts, phosphorous acid and its salts, piperalin, propanosine-sodium, proquinazid, pyrrolnitrine, quintozene, tecloftalam, tecnazene, triazoxide, trichlamide, zarilamid and 2,3 ,5 ,6-tetrachloro-4-(methylsulfonyl)-pyridine, N-(4-Chloro-2-nitrophenyl)-N- ethyl-4-methyl-benzenesulfonamide, 2-amino-4-methyl-N-phenyl-5- thiazolecarboxamide, 2-chloro-N-(2,3-dihydro- 1 , 1 ,3-trimethyl- lH-inden-4-yl)-3- pyridincarboxamide, 3 - [5 -(4-chlorophenyl)-2,3 -dimethylisoxazo lidin-3 -yl]pyridine,
cis- 1 -(4-chlorophenyl)-2-( IH-1 ,2,4-triazole- 1 -yl)-cycloheptanol, methyl 1 -(2,3- dihydro-2,2-dimethyl-lH-inden-l-yl)-lH-imidazole-5-carboxylate, 3,4,5-trichloro- 2,6-pyridinedicarbonitrile, Methyl 2-[[[cyclopropyl[(4- methoxyphenyl)imino]methyl]thio]methyl]-.alpha.-(methoxymethylene)- benzeneacetate, 4-Chloro-alpha-propynyloxy-N-[2-[3-methoxy-4-(2- propynyloxy)phenyl]ethyl]-benzeneacetamide, (2S)-N-[2-[4-[[3-(4-chlorophenyl)-2- propynyljoxy] -3 -methoxyphenyl] ethyl]- 3 -methyl-2- [(methylsulfonyl)amino] - butanamide, 5-chloro-7-(4-methylpiperidin- 1 -yl)-6-(2,4,6- trifluorophenyl)[l,2,4]triazolo[l,5-a]pyrimidine, 5-chloro-6-(2,4,6-trifluorophenyl)- N-[(lR)-l,2,2-trimethylpropyl][l,2,4]triazolo[l,5-a]pyrimidin-7-amine, 5-chloro-N- [(lR)-l,2-dimethylpropyl]-6-(2,4,6-trifluorophenyl)[l,2,4]triazolo[l,5-a]pyrimidin- 7-amine, N- [ 1 -(5 -bromo-3 -chloropyridin-2-yl)ethyl] -2,4-dichloronicotinamide, N-(5 - bromo-3-chloropyridin-2-yl)methyl-2,4-dichloronicotinamide, 2-butoxy-6-iodo-3- propyl-benzopyranon-4-one, N-{(Z)-[(cyclopropylmethoxy)imino][6- (difluoromethoxy)-2,3-difluorophenyl]methyl}-2-phenylacetamide, N-(3-ethyl-3,5,5- trimethyl-cyclohexyl)-3-formylamino-2-hydroxy-benzamide, 2-[[[[l-[3(lFluoro-2- phenylethyl)oxy]phenyl] ethylidene] amino] oxy]methyl] -alpha-(methoxyimino)-N- methyl-alphaE-benzeneacetamide, N-{2-[3-chloro-5-(trifluoromethyl)pyridin-2- yl]ethyl}-2-(trifluoromethyl)benzamide, N-(3',4'-dichloro-5-fluorobiphenyl-2-yl)-3- (difluoromethyl)- 1 -methyl- 1 H-pyrazole-4-carboxamide, 2-(2- { [6-(3 -chloro-2- methylphenoxy)-5-fluoropyrimidin-
4-yl]oxy}phenyl)-2-(methoxyimino)-N-methylacetamide , l-[(4- methoxyphenoxy)methyl]-2,2-dimethylpropyl-lH-imidazole-l- carboxylic acid, O- [ 1 -[(4-methoxyphenoxy)methyl]-2,2-dimethylpropyl]- lH-imidazole- 1 - carbothioic acid.
The composition according to the invention comprising a mixture of a compound of formula (I) with a bactericide compound may also be particularly advantageous. Examples of suitable bactericide mixing partners may be selected in the following list : bronopol, dichlorophen, nitrapyrin, nickel dimethyldithiocarbamate, kasugamycin, octhilinone, furancarboxylic acid, oxytetracycline, probenazole, streptomycin, tecloftalam, copper sulphate and other copper preparations.
The fungicidal compositions of the present invention can be used to curatively or preventively control the phytopathogenic fungi of crops. Thus, according to a further
aspect of the present invention, there is provided a method for curatively or preventively controlling the phytopathogenic fungi of crops characterised in that a fungicidal composition as hereinbefore defined is applied to the seed, the plant and/or to the fruit of the plant or to the soil in which the plant is growing or in which it is desired to grow.
The composition as used against phytopathogenic fungi of crops comprises an effective and non-phytotoxic amount of an active material of general formula (I).
The expression "effective and non-phytotoxic amount" means an amount of composition according to the invention which is sufficient to control or destroy the fungi present or liable to appear on the crops, and which does not entail any appreciable symptom of phytotoxicity for the said crops. Such an amount can vary within a wide range depending on the fungus to be controlled, the type of crop, the climatic conditions and the compounds included in the fungicidal composition according to the invention.
This amount can be determined by systematic field trials, which are within the capabilities of a person skilled in the art.
The method of treatment according to the present invention is useful to treat propagation material such as tubers or rhizomes, but also seeds, seedlings or seedlings pricking out and plants or plants pricking out. This method of treatment can also be useful to treat roots. The method of treatment according to the present invention can also be useful to treat the overground parts of the plant such as trunks, stems or stalks, leaves, flowers and fruits of the concerned plant.
Among the plants that can be protected by the method according to the present invention, mention may be made of cotton; flax; vine; fruit or vegetable crops such as Rosaceae sp. (for instance pip fruit such as apples and pears, but also stone fruit such as apricots, almonds and peaches), Ribesioidae sp., Juglandaceae sp., Betulaceae sp., Anacardiaceae sp., Fagaceae sp., Moraceae sp., Oleaceae sp., Actinidaceae sp., Lauraceae sp., Musaceae sp. (for instance banana trees and plantins), Rubiaceae sp., Theaceae sp., Sterculiceae sp., Rutaceae sp. (for instance lemons, oranges and grapefruit); Solanaceae sp. (for instance tomatoes), Liliaceae sp., Asteraceae sp. (for instance lettuces), Umbelliferae sp., Cruciferae sp., Chenopodiaceae sp., Cucurbitaceae sp., Papilionaceae sp. (for instance peas), Rosaceae sp. (for instance strawberries); major crops such as Graminae sp. (for instance maize, lawn or cereals such as wheat, rice, barley and triticale), Asteraceae sp. (for instance sunflower), Cruciferae sp. (for instance colza), Fabacae sp. (for instance peanuts), Papilionaceae sp. (for instance soybean), Solanaceae sp. (for
instance potatoes), Chenopodiaceae sp. (for instance beetroots); horticultural and forest crops; as well as genetically modified homologues of these crops.
Among the diseases of plants or crops that can be controlled by the method according to the present invention, mention may be made of : Powdery mildew diseases such as :
Blumeria diseases, caused for example by Blumeria graminis; Podosphaera diseases, caused for example by Podosphaera leucotricha; Sphaerotheca diseases, caused for example by Sphaerotheca fuliginea; Uncinula diseases, caused for example by Uncinula necator; Rust diseases such as :
Gymnosporangium diseases, caused for example by Gymnosporangium sabinae;
Hemileia diseases, caused for example by Hemileia vastatrix; Phakopsora diseases, caused for example by Phakopsora pachyrhizi or Phakopsora meibomiae;
Puccinia diseases, caused for example by Puccinia recondita; Uromyces diseases, caused for example by Uromyces appendiculatus; Oomycete diseases such as :
Bremia diseases, caused for example by Bremia lactucae; Peronospora diseases, caused for example by Peronospora pisi or P. brassicae;
Phytophthora diseases, caused for example by Phytophthora infestans; Plasmopara diseases, caused for example by Plasmopara viticola; Pseudoperonospora diseases, caused for example by Pseudoperonospora humuli or Pseudoperonospora cubensis; Pythium diseases, caused for example by Pythium ultimum;
Leafspot, leaf blotch and leaf blight diseases such as :
Alternaria diseases, caused for example by Alternaria solani; Cercospora diseases, caused for example by Cercospora beticola; Cladiosporum diseases, caused for example by Cladiosporium cucumerinum; Cochliobolus diseases, caused for example by Cochliobolus sativus;
Colletotrichum diseases, caused for example by Cottetotrichum lindemuthan ium ;
Cycloconium diseases, caused for example by Cycloconium oleaginum; Diaporthe diseases, caused for example by Diaporthe citri; Elsinoe diseases, caused for example by Elsinoe fawcettii;
Gloeosporium diseases, caused for example by Gloeosporium laeticolor;
Glomerella diseases, caused for example by Glomerella cingulata;
Guignardia diseases, caused for example by Guignardia bidwelli;
Leptosphaeria diseases, caused for example by Leptosphaeria maculans; Leptosphaeria nodorum; Magnaporthe diseases, caused for example by Magnaporthe grisea;
Mycosphaerella diseases, caused for example by Mycosphaerella graminicola; Mycosphaerella arachidicola; Mycosphaerella βjiens is;
Phaeosphaeria diseases, caused for example by Phaeosphaeria nodorum;
Pyrenophora diseases, caused for example by Pyrenophora teres; Ramularia diseases, caused for example by Ramularia collo-cygni;
Rhynchosporium diseases, caused for example by Rhynchosporium secalis;
Septoria diseases, caused for example by Septoria apii or Septoria lycopercisi;
Typhula diseases, caused for example by Typhula incarnata;
Venturia diseases, caused for example by Venturia inaequalis; Root and stem diseases such as :
Corticium diseases, caused for example by Corticium graminearum;
Fusarium diseases, caused for example by Fusarium oxysporum;
Gaeumannomyces diseases, caused for example by Gaeumannomyces graminis; Rhizoctonia diseases, caused for example by Rhizoctonia solani;
Tapesia diseases, caused for example by Tapesia acuformis;
Thielaviopsis diseases, caused for example by Thielaviopsis basicola; Ear and panicle diseases such as :
Alternaria diseases, caused for example by Alternaria spp.; Aspergillus diseases, caused for example by Aspergillus flavus;
Cladosporium diseases, caused for example by Cladosporium spp.;
Claviceps diseases, caused for example by Claviceps purpurea;
Fusarium diseases, caused for example by Fusarium culmorum;
Gibberella diseases, caused for example by Gibberella zeae; Monographella diseases, caused for example by Monographella nivalis;
Smut and bunt diseases such as :
Sphacelotheca diseases, caused for example by Sphacelotheca reiliana;
Tilletia diseases, caused for example by Tilletia caries;
Urocystis diseases, caused for example by Urocystis occulta; Ustilago diseases, caused for example by Ustilago nuda;
Fruit rot and mould diseases such as :
Aspergillus diseases, caused for example by Aspergillus flavus;
Botrytis diseases, caused for example by Botrytis cinerea;
Penicillium diseases, caused for example by Penicillium expansum;
Sclerotinia diseases, caused for example by Sclerotinia sclerotiorum; Verticilium diseases, caused for example by Verticilium alboatrum;
Seed and soilborne decay, mould, wilt, rot and damping-off diseases :
Fusarium diseases, caused for example by Fusarium culmorum;
Phytophthora diseases, caused for example by Phytophthora cactorum;
Pythium diseases, caused for example by Pythium ultimum; Rhizoctonia diseases, caused for example by Rhizoctonia solani;
Sclerotium diseases, caused for example by Sclerotium rolfsii;
Microdochium diseases, caused for example by Microdochium nivale; Canker, broom and dieback diseases such as :
Nectria diseases, caused for example by Nectria galligena; Blight diseases such as :
Monilinia diseases, caused for example by Monilinia laxa; Leaf blister or leaf curl diseases such as :
Taphrina diseases, caused for example by Taphrina deformans; Decline diseases of wooden plants such as : Esca diseases, caused for example by Phaemoniella clamydospora;
Diseases of flowers and Seeds such as :
Botrytis diseases, caused for example by Botrytis cinerea; Diseases of tubers such as :
Rhizoctonia diseases, caused for example by Rhizoctonia solani.
The fungicide composition according to the present invention may also be used against fungal diseases liable to grow on or inside timber. The term "timber" means all types of species of wood, and all types of working of this wood intended for construction, for example solid wood, high-density wood, laminated wood, and plywood. The method for treating timber according to the invention mainly consists in contacting one or more compounds of the present invention, or a composition according to the invention; this includes for example direct application, spraying, dipping, injection or any other suitable means.
The dose of active material usually applied in the treatment according to the present invention is generally and advantageously between 10 and 800 g/ha, preferably
between 50 and 300 g/ha for applications in foliar treatment. The dose of active substance applied is generally and advantageously between 2 and 200 g per 100 kg of seed, preferably between 3 and 150 g per 100 kg of seed in the case of seed treatment. It is clearly understood that the doses indicated above are given as illustrative examples of the invention. A person skilled in the art will know how to adapt the application doses according to the nature of the crop to be treated.
The fungicidal composition according to the present invention may also be used in the treatment of genetically modified organisms with the compounds according to the invention or the agrochemical compositions according to the invention. Genetically modified plants are plants into whose genome a heterologous gene encoding a protein of interest has been stably integrated. The expression "heterologous gene encoding a protein of interest" essentially means genes which give the transformed plant new agronomic properties, or genes for improving the agronomic quality of the transformed plant.
The compositions according to the present invention may also be used for the preparation of composition useful to curatively or preventively treat human and animal fungal diseases such as, for example, mycoses, dermatoses, trichophyton diseases and candidiases or diseases caused by Aspergillus spp., for example Aspergillus fumigatus.
The aspects of the present invention will now be illustrated with reference to the following tables of compounds and examples. The following Tables A to V illustrate in a non-limiting manner examples of fungicidal compounds according to the present invention. In the following Examples, M+l (or M-I) means the molecular ion peak, plus or minus 1 a.mu. (atomic mass units) respectively, as observed in mass spectroscopy.
Table 1
Compound R1 R2 R3 n X1 X2 X3 W R4 R5 R6 [M+l]
K)
1-1 H Me H 2 H H - C H Me Me 349
1-2 H Me H 3 H H H C H Me Me 363
1-3 H Me Me 3 H H H C H Me Me 377
Table 2
Compound R1 R2 R3 n X1 X2 X3 W R4 R5 R6 [M+l]
2-1 H Me H 2 H H - C H Me Me 291 K)
VO
2-2 H Me H 3 H H H C H Me Me 305
2-3 H Me Me 3 H H H C H Me Me 319
2-4 Cyclop Me H 3 H Me H Si Me Me Me 389
2-5 H Me H 3 Me Me H Si Me Me Me 363
2-6 H Me H 3 H Me H Si Me Me Me 349
Table 3
Compound R1 R2 R3 n X1 XΛ W R4 Rs R" [M+l]
3-1 H Me H 2 H H - C H Me Me 352
3-2 H Me H 3 H H H C H Me Me 366 O
3-3 H Me H 3 Me Me H Si Me Me Me 410
3-4 H Me Me 3 H H H C H Me Me 380
3-5 H Me H 3 H Me H Si Me Me Me 410
Table 4
Compound R1 R2 R3 n X1 X2 x- 5 W R4 R5 R6 [M+l]
4-1 H Me H 2 H H - C H Me Me 292
W
4-2 H Me H 3 H H H C H H Me 292
4-3 H Me H 3 H H H Si Me Me Me 336
4-4 H Me H 3 H Me H C Me Me Me 334
4-5 H Me H 3 H Me H Si Me Me Me 350
4-6 H Me H 3 Me Me H Si Me Me Me 364
4-7 H Me Me 3 H H H C H Me Me 320
4-8 Cyclopropyl Me H 3 H Me H Si Me Me Me 390
4-9 H (R)-Me H 3 H (R)-Me H C Me Me Me 334
Compound R1 R2 R3 n X1 X2 X3 W R4 R5 R6 [M+l]
4-10 H (R)-Me H 3 H (S)-Me H C Me Me Me 334
4-11 H (S)-Me H 3 H (S)-Me H C Me Me Me 334
4-12 H (S)-Me H 3 H (R)-Me H C Me Me Me 334
Table 5
Compound R1 R2 R3 n X1 X2 X3 W R4 R5 R6 [M+l]
5-1 H Me H 2 H H - C H Me Me 274
5-2 H Me H 3 H H H C H H Me 274
5-3 H Me H 3 H Me H C Me Me Me 316
5-4 H Me H 3 H Me H Si Me Me Me 332
5-5 H Me H 3 Me Me H Si Me Me Me 346
5-6 H Me Me 3 H H H C H Me Me 302
Table 6
Compound R1 R2 R3 n X1 X2 X3 W R4 R5 R6 [M+l]
4-
6-1 H Me H Me Me H Si Me Me Me 340
Table 7
Compound R1 R2 R3 X1 X2 X3 W R4 R5 R6 [M+l]
7-1 H Me H 2 H H - C H Me Me 256
7-2 H Me H 3 H Me H C Me Me Me 298
Ul
7-3 H Me H 3 H Me H Si Me Me Me 314
7-4 H Me H 3 Λe Me H Si Me Me Me 328
7-5 H Me Me 3 H H H C H Me Me 284
7-6 C Cvyccllooϋpropyl Me H H Me H Si Me Me Me 354
Table 8
Compound R1 Rz R3 X1 X2 XΛ W R4 Rs R6 [M+l]
8-1 H Me H 3 H Me H C Me Me Me 296
8-2 H Me H 3 H Me H Si Me Me Me 312
8-3 H Me H 3 Me Me H Si Me Me Me 326
8-4 Cyclopropyl Me H 3 H Me H Si Me Me Me 352
Table 9
Compound R1 Rz R3 n X1 X2 XΛ W R4 Rs R" [M+l]
9-1 H Me H 2 H H - C H Me Me 309
9-2 H Me H 3 H H H C H H Me 309
9-3 H Me H 3 H Me H Si Me Me Me 367 -4
9-4 H Me H 3 Me Me H Si Me Me Me 381
9-5 H Me Me 3 H H H C H Me Me 337
9-6 Cyclopropyl Me H 3 H Me H Si Me Me Me 407
6.03 (d, IH)
4.14 (m, IH)
2.71 (m, 3H)
1.58 (m, IH)
1.40 (m, 2H)
9-7 H Me H 3 H Me H C Me Me Me 1.24 (m, IH)
1.21 (d, 3H)
1.08 (dd, IH)
0.97 (d, 3H)
0.89 (s, 9H)
Table 10
Compound R1 R2 R3 n X1 X2 X3 W R4 R5 R6 [M+l]
10-1 H Me H 2 H H - C H Me Me 291
10-2 H Me H 3 H H H C H H Me 291
10-3 H Me H 3 H H H Si Me Me Me 335
10-4 H Me H 3 H Me H C Me Me Me 333
10-5 H Me H 3 H Me H Si Me Me Me 349
10-6 H Me H 3 Me Me H Si Me Me Me 363
10-7 H Me Me 3 H H H C H Me Me 319
10-8 ( ^vclonronv 1 Me H 3 H Me H Si Me Me Me 389
Table 11
Compound R1 R2 R3 n X1 X2 X3 W R R5 R6 [M+l]
11-1 H Me H 2 H H - C H Me Me 304
11-2 H Me H 3 H H H C H Me Me 318
VO
11-3 H Me H 3 H Me H C Me Me Me 346
11-4 H Me Me 3 H H H C H Me Me 332
11-5 H Me H 3 Me Me H Si Me Me Me 377
11-6 H Me H 3 H Me H Si Me Me Me 363
Table 12
Compound R1 R2 R3 n X1 X2 X3 W R4 R5 R6 [M+l]
12-1 H Me H 2 H H - C H Me Me 352
12-2 H Me H 3 H H H C H Me Me 366
12-3 H Me Me 3 H H H C H Me Me 380
Table 13
Compound R R2 R3 X1 X2 X3 W R4 R5 R6 [M+l]
13-1 H Me H 2 H H - C H Me Me 240
13-2 H Me H 3 H H H C H Me Me 254
13-3 H Me H 3 Me Me H Si Me Me Me 312
13-4 H Me Me 3 H H H C H Me Me 268
13-5 H Me H 3 Me Me H Si Me Me Me 312
Table 14
Compound R1 Rz R3 X1 X2 X3 W R4 Rs R6 [M+l]
14-1 H Me H 2 H H - C H Me Me 292
14-2 H Me H 3 H H H C H Me Me 306
14-3 Cyclopropyl Me H 3 H Me H Si Me Me Me 390
14-4 H Me H 3 Me Me H Si Me Me Me 364
Table 15
Compound R1 R2 R3 X1 X2 XΛ W R4 Rs R6 [M+l]
15-1 H Me H 2 H H - C H Me Me 336
15-2 H Me H 3 H H H C H Me Me 350
15-3 H Me H 3 Me Me H Si Me Me Me 406
15-4 H Me Me 3 H H H C H Me Me 364
Table 16
Compound R1 R2 R3 X1 X2 X3 W R4 Rs R6 [M+l]
16-1 H Me H 2 H H - C H Me Me 238
16-2 H Me H 3 H H H C H Me Me 252
16-3 H Me H 3 Me Me H Si Me Me Me 310
16-4 H Me H 3 Me Me H Si Me Me Me 310
Table 17
Compound R R R3 X1 X2 X3 W R4 R5 R6 [M+l]
17-1 H Me H 2 H H - C H Me Me 224
17-2 H Me H 3 H H H C H Me Me 238
17-3 H Me H 3 H Me H C Me Me Me 266
17-4 H Me H 3 Me Me H Si Me Me Me 296
17-5 H Me H 3 Me Me H Si Me Me Me 296
Compound R1 R2 R3 n X1 X2 R10 R 11 R 12 [M+l]
19-1 H Me H 1 H H H H 317
Table 20
Compound R1 R2 R3 n X1 X2 R 10 R 11 R 12 [M+l]
20-1 H Me H H H H H 378
Table 21
Compound R1 R2 R3 n X1 X2 R10 R11 R12 [M+l]
21-1 H Me H 1 H - H H H 318
21-2 H Me H 2 H H H H H 332
21-3 H Me H 2 H H Me Me Me 388
Table 22
Compound R1 R2 R3 n X1 X2 R10 R11 R12 [M+l]
Ul
O
22-1 H Me H 1 H - H H H 300
22-2 H Me H 2 H H H H H 314
22-3 H Me H ?, H H Me Me Me 370
Table 23
Compound R1 R2 R3 n X1 X2 R10 R11 R12 [M+l]
23-1 H Me H 1 H H H H 296
Table 24
Compound R1 R2 R3 n X1 X2 R 10 R 11 R 12 [M+l]
Ul K>
24-1 H Me H 1 H - H H H 282
24-2 H Me H 2 H H Me Me Me 352
Table 25
Compound R1 R2 R3 n X1 X2 R10 R11 R12 [M+l]
25-1 H Me H 1 H - H H H 335 Ul
25-2 H Me H 2 H H H H H 349
25-3 H Me H 2 H H Me Me Me 405
Compound R1 R2 R3 n X1 X2 R 10 R 11 R 12 [M+l]
27-1 H Me H 1 H H H H 330
Ul
Table 28
Compound R1 R2 R3 n X1 X2 R 10 R 11 R 12 [M+l]
28-1 H Me H 1 H - H H H 378
Ul
28-2 H Me H 2 H H Me Me Me 448
Table 29
Compound R1 R2 R3 n X1 X2 R 10 R 11 R 12 [M+l]
29-1 H Me H 1 H - H H H 266
-4
29-2 H Me H 2 H H Me Me Me 336
Table 30
Compound R1 R2 R3 n X1 X2 R 10 R 11 R 12 [M+l]
30-1 H Me H 1 H - H H H 318 Ul
30-2 H Me H 2 H H Me Me Me 388
Compound R1 R2 R3 n X1 X R 10 R 11 R 12 [M+l]
32-1 H Me H 1 H - H H H 264 o
32-2 H Me H 2 H H Me Me Me 334
Table 33
33-2 H Me H 2 H H Me Me Me 320
Table 34
Compound R1 R2 R3 n X1 X2 X3 W R4 R5 R6 [M+l]
34-1 H Me H 3 Me Me H Si Me Me Me 340 34-2 H Me H 3 H Me H Si Me Me Me 326
Table 35
Compound R1 R2 R3 X1 X2 X3 W R4 R5 R6 [M+l]
35-1 H Me H 3 Me Me H Si Me Me Me 341
35-2 H Me H 3 H Me H Si Me Me Me 327
Examples of process for the preparation of the compound of general formula (I)
Example 1 : Preparation of N-[l,3-dimethyl-4-(trimethylsilyl)butyll-5-fluoro- l,3-dimethyl-lH-pyrazole-4-carboxamide (Compound 7-3) Preparation of 4-methyl-5-(trimethylsilyl)pentan-2-one
A solution of 8,00 g pent-3-en-2-one in 50 mL dry diethyl ether was dropped to a solution of 73.5 mL of chloro[(trimethylsilyl)methyl]magnesium (1.0M in diethyl ether) and 955 mg of CuBr at -5°C under Argon. The mixture was stirred for 1 hour at room temperature. An aqueous work-up followed by silica gel chromatography yielded 5,20 g (43%) of a colourless oil. [M+l] = 173.
Preparation of 4-methyl-5-(trimethylsilyl)pentan-2-amine hydrochloride
A solution of 7,4 g 4-methyl-5-(trimethylsilyl)pentan-2-one, 29,8 g ammonium acetate, and 5 g molecular sieve 3A in 500 mL Methanol was stirred for 4 hours at room temperature. 4,86 g sodium cyanoborohydride was added. The reaction was completed over night at room temperature. The crude reaction mixture was evaporated. Extraction with diethyl ether, basic aqueous treatment and evaporation yielded 2.20 g (26%) of a colourless solid. [M-HCl+1] = 174.
Preparation of N-[l,3-dimethyl-4-(trimethylsilyl)butyl]-5-fluoro-l,3-dimethyl-lH- pyrazole-4-carboxamide
91.6 mg of 5-fluoro-l,3-dimethyl-lH-pyrazole-4-carbonyl chloride was added to a solution of 173 mg 4-methyl-5-(trimethylsilyl)pentan-2-amine and 71.7 mg of potassium carbonate in 3 mL methylene chloride. The reaction mixture was stirred 2 hours at room temperature. Aqueous work-up, extraction with ethyl acetate and silica gel chromatography yielded 115 mg (67%) of a colourless oil. [M+l] = 314.
Example 2 : Preparation of N-(3-cvclohexyl-l-methylpropyD-3- fdifluoromethyl)-l-methyl-lH-pyrazole-4-carboxamide (Compound 22-2)
Preparation of 3-cyclohexyl-l -methylpropyl methanesulfonate A solution of 3.53 g methanesulfonyl chloride in 100 mL methylene chloride was added to a solution of 4.38 g 4-cyclohexylbutan-2-ol in 200 mL methylene chloride. The reaction mixture was stirred over night. Aqueous work-up yielded 7.06 g (96%) of a colourless oil.
Preparation of2-(3-cyclohexyl-l-methylpropyl)-lH-isoindole-l,3(2H)-dione
A solution of 7.00 g 3-cyclohexyl-l -methylpropyl methanesulfonate in 20 mL DMF was added to a solution of 5.14 g phthalimide and 7.43 g of potassium carbonate in 50 mL DMF at room temperature. The reaction mixture was stirred at 800C over night. Aqueous work-up followed by silica gel filtration yielded 4.95 g (58%) of a colourless oil. [M+l] = 286.
Preparation of 4-cyclohexylbutan-2-amine
A solution of 4.60 g 2-(3-cyclohexyl-l-methylpropyl)-lH-isoindole-l,3(2H)- dione and 7.26 g of hydrazine hydrate in 60 mL ethanol was heated to 700C. The white precipitation was filtered off after cooling to room temperature. The mixture was washed with water and evaporated. 920 mg (37%) of a colourless oil were obtained.
Preparation of N-(3-cyclohexyl-l-methylpropyl)-3-(difluoromethyl)-l-methyl-lH- pyrazole-4-carboxamide
A solution of 489 mg PS DCC [N-Cyclohexylcarbodiimide^'-methyl polystyrene], 90.7 mg 3 -(difluoromethyl)-l -methyl- lH-pyrazole-4-carboxylic acid , 69.6 mg 1-hydroxybenzotriazole (HOBT) in 3.2 mL acetonitrile was heated for 5 min to 1000C in the microwave oven. After cooling the reaction mixture 200 mg of Amberlist A21 were added and the mixture was heated again to 1000C in the microwave oven. The cold reaction mixture was filtered, the resin was washed with
methylene chloride. Silica gel chromatography yielded 89 mg (52%) of a brownish oil.
[M+l] = 314.
Example 3 : Preparation of 4-(difluoromethyl)-2-methyl-N-[l-methyl-4- (trimethylsilyl)butyll-l.,3-thiazole-5-carboxamide (Compound 10-3)
A solution of 133 mg of 4-(difluoromethyl)-2-methyl-l,3-thiazole-5- carboxylic acid, 110 mg 5-(trimethylsilyl)pentan-2-amine, 354 mg of PyBroP [bromo-tris-pyrrolidino-phosphonium hexafiuorophosphate], and 142 mg N,N'- diisopropylamine in 10 mL acetonitrile was stirred over night at room temperature. Aqueous work-up [extraction with 1.0M NaOH], and silica gel chromatography yielded 130 mg (53%) of a colourless oil. [M+l] = 335.
Examples of biological activity of the compound of general formula (I)
Example A : in vivo test on Alternaria brassicae (Leaf spot of crucifers)
The active ingredient tested is prepared by homogenisation in DMSO (5 % of the final volume), acetone (10% of the final volume) and tween 80 10% (5 μl/mg active ingredient).
Radish plants (Pernot variety) in starter cups, sown on a 50/50 peat soil-pozzolana substrate and grown at 18-200C, are treated at the cotyledon stage by spraying with the aqueous suspension described above.
Plants, used as controls, are treated with an aqueous solution not containing the active material.
After 24 hours, the plants are contaminated by spraying them with an aqueous suspension of Alternaria brassicae spores (40,000 spores per cm3). The spores are collected from a 12 to 13 days-old culture.
The contaminated radish plants are incubated for 6-7 days at about 18°C, under a humid atmosphere.
Grading is carried out 6 to 7 days after the contamination, in comparison with the control plants.
Under these conditions, good (at least 70%) or total protection is observed at a dose of 500 ppm with the following compounds : 1-2, 2-2, 2-5, 3-2, 3-5, 4-3 ,4-4,
4-5, 4-6, 5-4, 5-5, 5-7, 6-1, 7-1, 7-3, 7-4, 7-6, 9-3, 10-3, 10-4, 10-5, 10-6, 10-8, 11-1,
11-2, 12-2, 13-1, 13-3, 13-5, 15-2, 15-3, 16-4, 19-1, 20-1, 21-1, 21-2, 22-2, 22-3, 23- 1, 24-1, 24-2, 25-1, 25-3, 26-2, 27-1, 28-2, 30-1, 30-2, 31-1, 32-1 and 33-2.
Under these conditions, 4-dichloro-N-octylisothiazole-5-carboxamide disclosed in the US patent US 5,240,951 (see compound 23) did not show any activity against Alternaria brassicae.
Example B : in vivo test on Botrvtis cinerea (cucumber Grey mould)
The active ingredient tested is prepared by homogenisation in DMSO (5 % of the final volume), acetone (10% of the final volume) and tween 80 10% (5 μl/mg active ingredient).
Cucumber plants (Marketer variety) in starter cups, sown on a 50/50 peat soil-pozzolana substrate and grown at 18- 200C, are treated at the cotyledon ZI l stage by spraying with the aqueous suspension described above. Plants, used as controls, are treated with an aqueous solution not containing the active material. After 24 hours, the plants are contaminated by depositing drops of an aqueous suspension oϊBotrytis cinerea spores (150,000 spores per ml) on upper surface of the leaves. The spores are collected from a 15-day-old culture and are suspended in a nutrient solution composed of :
- 20 g/L of gelatin - 50 g/L of cane sugar
- 2 g/L of NH4NO3
- 1 g/L of KH2PO4
The contaminated cucumber plants are settled for 5/7 days in a climatic room at 15-110C (day/night) and at 80% relative humidity. Grading is carried out 5/7 days after the contamination, in comparison with the control plants. Under these conditions, good (at least 70%) or total protection is observed :
- at a dose of 500 ppm with the following compounds : 2-1, 2-2, 3-2, 4-3, 4-4, 4-5, 4-7, 4-11, 5-4, 5-5, 5-6, 5-7, 7-1, 7-2, 7-5, 7-6, 9-3, 10-3, 10-4, 10-5, 11-2, 12-2, 13-2, 15-1, 15-2, 18-1, 19-1, 20-1, 21-1, 21-2, 22-2, 24-1, 25-2, 26-2, 27-1, 28-1, 29- 1, 30-1, 31-1, 33-1 and 33-2; and
- at a dose of 250 ppm for the following compound : 2-6.
Under these conditions, N-isopropyl-4-methyl-l,2,3-thiadiazole-5- carboxamide disclosed in the International Patent Application WO 96/29871 (see compound 1-89), N-sec-butyl-2,5-dimethyl-3-furamide disclosed in GB patent application GB 1387652 (see compound 3) and 4-dichloro-N-octylisothiazole-5- carboxamide disclosed in the US patent US 5,240,951 (see compound 23) did not show any activity against Botrytis cinerea.
Example C : in vivo test on Pyrenophora teres (Barley Net blotch)
The active ingredient tested is prepared by homogenisation in DMSO (5 % of the final volume), acetone (10% of the final volume) and tween 80 10% (5 μl/mg active ingredient).
Barley plants (Express variety) in starter cups, sown on a 50/50 peat soil-pozzolana substrate and grown at 12°C, are treated at the 1-leaf stage (10 cm tall) by spraying with the aqueous suspension described above. Plants, used as controls, are treated with an aqueous solution not containing the active material. After 24 hours, the plants are contaminated by spraying them with an aqueous suspension of Pyrenophora teres spores (12,000 spores per ml). The spores are collected from a 12-day-old culture .The contaminated barley plants are incubated for 24 hours at about 200C and at 100% relative humidity, and then for 12 days at 80% relative humidity. Grading is carried out 12 days after the contamination, in comparison with the control plants. Under these conditions, good (at least 70%) or total protection is observed :
- at a dose of 500 ppm with the following compounds : 2-1, 2-2, 2-4, 2-5, 3-1, 3-2, 3-5, 4-5, 4-6, 4-8, 4-12, 5-4, 5-5, 5-7, 7-1, 7-2, 7-3, 7-4, 7-6, 8-4, 9-3, 9-4, 10-5, 10-6, 10-8, 11-5, 12-2, 14-4, 15-2, 16-4, 17-5, 19-1, 21-2, 21-3, 22-2, 22-3, 24-1, 24- 2, 25-2, 25-3, 26-2, 26-3, 27-1, 28-2, 29-2, 30-1, 30-2, 31-1, 32-1, 33-1, 33-2 and 35- l;
- at a dose of 330 ppm for the following compound : 11-3;
- at a dose of 250 ppm for the following compound : 2-6; and - at a dose of 500 g/ha with the following compounds : 4-3, 4-4, 10-3, 10-4,
21-1, 22-1, 25-1 and 26-1.
Under these conditions, N-sec-butyl-2,5-dimethyl-3-furamide disclosed in GB patent application GB 1387652 (see compound 3) did not show any activity against Pyrenophora teres.
Example D : in vivo test on Svhaerotheca fulisinea (powdery mildew)
The active ingredient tested is prepared by homogenisation in DMSO (5 % of the final volume), acetone (10% of the final volume) and tween 80 10% (5 μl/mg active ingredient).
Gherkin plants (Vert petit de Paris variety) in starter cups, sown on a 50/50 peat soil-pozzolana substrate and grown at 20°C/23°C, are treated at cotyledon ZI l stage by spraying with the aqueous suspension described above. Plants, used as controls, are treated with an aqueous solution not containing the active material.
After 24 hours, the plants are contaminated by spraying them with an aqueous suspension of Sphaerotheca fuliginea spores (100 000 spores per ml). The spores are collected from a contaminated plants .The contaminated gerkhin plants are incubated at about 20°C/25°C and at 60/70% relative humidity. Grading (% of efficacy) is carried out 21 days after the contamination, in comparison with the control plants. Under these conditions, good (at least 70%) or total protection is observed :
- at a dose of 500 ppm with the following compounds : 2-1, 2-4, 2-5, 3-1, 3-3, 3-5, 4-3, 4-4, 4-5, 4-6, 4-8, 4-11, 4-12, 5-4, 5-5, 5-6, 5-7, 7-2, 7-3, 7-4, 7-5, 7-6, 8-4, 9-3, 10-3, 10-4, 10-5, 10-6, 13-3, 13-5, 14-4, 15-3, 21-1, 21-2, 21-3, 22-1, 22-2, 22-3, 24-2, 25-1, 25-2, 25-3, 26-1, 26-2, 26-3, 28-2, 29-2, 30-2, 33-1 and 33-2; and
- at a dose of 250 pmm for the following compound : 2-6.
Under these conditions, N-isopropyl-4-methyl-l,2,3-thiadiazole-5- carboxamide disclosed in the International Patent Application WO 96/29871 (see compound 1-89) and N-sec-butyl-2,5-dimethyl-3-furamide disclosed in GB patent application GB 1387652 (see compound 3) did not show any activity against
Sphaerotheca fuliginea.
Claims
1. A compound of general formula (I)
- Het represents 5-membered non-fused heterocycle with one, two or three heteroatoms which may be the same or different, Het being linked by a carbon atom and being at least substituted in ortho position;
- n is 2, 3 or 4;
- R1 is chosen as being a hydrogen atom, a Ci-Cό-alkyl group or a C3-C7- cycloalkyl;
- R2 is chosen as being a Ci-Cό-alkyl group; - R3 is chosen as being a hydrogen atom or a Ci-C6-alkyl group;
- each X is chosen, independently of the others, as being a hydrogen atom or a Ci-Ce-alkyl group;
- W represents a carbon atom or a silicon atom;
- R4, R5 and R6 are chosen, independently of each other, as being a hydrogen atom, a halogen atom, or a Ci-Cό-alkyl group, at least two of the substituents R4, R5 and R6 being different from a hydrogen atom; or two of the substituents R4, R5 and R6 may together form a 3-, 4-, 5- or 6- membered non aromatic cycle optionally substituted with a Ci-Cό-alkyl group; as well as its salts, N-oxydes, metallic complexes, metalloidic complexes and optically active isomers with the exception of :
5-(dimethylamino)-N-(l,5-dimethylhexyl)-4-methyl-4H-l,2,4-triazole-3- carboxamide;
N-(l,5-dimethylhexyl)-l-[4-[[(l,5-dimethylhexyl)amino]sulfonyl]phenyl]-4-[[4- [ [( 1 ,5 -dimethylhexyl)amino] sulfonyljphenyl] azo] -5 -hydroxy- 1 H-pyrazole-3 - carboxamide;
5 -amino-N-( 1 ,5 -dimethylhexyl)-3 - {5 - [3 -(trifluoromethyl)phenyl] -2- furyl} isoxazole-4-carboxamide; - 5-amino-3-(5-bromothien-2-yl)-N-(l,5-dimethylhexyl)isoxazole-4-carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(5-methylthien-2-yl)isoxazole-4-carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(3-methylthien-2-yl)isoxazole-4-carboxamide;
5-amino-3-[5-(3-chlorophenyl)-2-furyl]-N-(l,5-dimethylhexyl)isoxazole-4- carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(5-methyl-2-furyl)isoxazole-4-carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(2-furyl)isoxazole-4-carboxamide;
- 5-amino-3-(l-benzofuran-3-yl)-N-(l,5-dimethylhexyl)isoxazole-4-carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-(5-ethylthien-2-yl)isoxazole-4-carboxamide; - 5-amino-3-(5-chloro-2-furyl)-N-(l ,5-dimethylhexyl)isoxazole-4-carboxamide;
5-amino-N-(l,5-dimethylhexyl)-3-(6-methoxypyridin-3-yl)isoxazole-4- carboxamide;
- 5-amino-N-(l,5-dimethylhexyl)-3-pyridin-3-ylisoxazole-4-carboxamide;
- 5-(4-chlorophenyl)-l-(2,4-dichlorophenyl)-N-(l,4-dimethylpentyl)- 4-methyl-lH-pyrazole-3-carboxamide; and
- 5-(4-chlorophenyl)-l-(2,4-dichlorophenyl)-N-(l,5-dimethylhexyl)- 4-methyl-lH-pyrazole-3-carboxamide.
2. A compound according to claim 1, characterised in that Het is chosen as being 2-furan, 3-furan, 4,5-dihydro-3-furan, 2-thiophene, 3-thiophene, 2-pyrrole, 3- pyrrole, 5-oxazole, 4-oxazole, 5-thiazole, 4-thiazole, 5-pyrazole, 4-pyrazole, 3- pyrazole, 3-isoxazole, 4-isoxazole, 5-isoxazole, 3-isothiazole, 4-1,2,3-triazole, 4- thiadiazole or 5-thiadiazole.
3. A compound according to claim 1 or 2, characterised in that each substituent of Het is chosen, independently of the others, as being a hydrogen atom, a halogen atom, an amino group, a cyano group, a Ci-C4-alkylamino, a di-(Ci -Chalky l)amino, a Ci-C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a C1-C4- halogenoalkoxy having 1 to 5 halogen atoms, a nitro group, a Ci-C4-alkyloxy, a Ci- C4-alkylthio, a Ci-C4-alkylsulphonyl, a phenyl optionally substituted by a halogen atom or a Ci-C4-alkyl or a pyridyl optionally substituted by a halogen atom or a Ci- C4-alkyl, a di(Ci-C4-alkyl)aminosulphonyl, a Ci-Co-alkylcarbonyl, a phenylsulphonyl optionally substituted by a halogen atom or a Ci-C4-alkyl, or a benzoyl optionally substituted by a halogen atom or a Ci-C4-alkyl, a carbamoyl group or an a Ci-Cs-alkylcarbonylamino, a C2-C6-alkenyl, a C3-C6-cycloalkyl, a Ci- C4-alkylthio-Ci-C4-alkyl, a Ci-C4-halogenoalkylthio-Ci-C4-alkyl having 1 to 5 halogen atoms, a Ci-C4-alkoxy-Ci-C4-alkyl or a Ci-C4-halogenoalkoxy-Ci-C4-alkyl having 1 to 5 halogen atoms.
4. A compound according to any of the claims 1 to 3, characterised in that Het is substituted in ortho position by a substituent chosen as being a halogen atom, a Ci-
C4-alkyl, a Ci-C4-halogenoalkyl having 1 to 5 halogen atoms, a C1-C4- halogenoalkoxy having 1 to 5 halogen atoms or a Ci-C4-alkyloxy;
5. A compound according to any of the claims 1 to 4, characterised in that R2 is chosen as being a methyl group.
6. A compound according to any of the claims 1 to 5, characterised in that n is 3.
7. A compound according to any of the claims 1 to 6, characterised in that R4, R R55 aanndd RR66 aarree be chosen, independently of each other as being a hydrogen atom or a methyl group.
8. A compound according to any of the claims 1 to 7, characterised in that each substituent X, which may the same or different, is chosen as being a hydrogen atom or a methyl group.
9. A compound of general formula (I-a)
- Het, R1, R2, R3, R4, R5 and R6 are as defined in claim 1 ; and
- X1 and X2 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
10. A compound of general formula (I-b)
- Het, R1, R2, R3, R4, R5 and R6 are as defined in claim 1; and
- X1, X2 and X3 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
11. A compound of general formula (I-c)
- Het, R1, R2, R3, R4, R5 and R6 are as defined in claim 1; and
- X1, X2, X3 and X4 are chosen, independently of each other, as being a hydrogen atom or a methyl group.
12. A process for the preparation of compound of general formula (I) as defined in any of the claims 1 to 11, which comprises reacting an alkyl-amine derivative of general formula (II) or one of its salt
in which R1, R2, R3, X, n, W, R4, R5 and R6 are as defined in claim 1 ; with a carboxylic acid derivative of the general formula (III)
-Het is as defined in claim 1 ; and
- L1 is a leaving group chosen as being a halogen atom, a hydroxyl group, - OR54, -OCOR54, R54 being a Ci-C6 alkyl, a Ci-C6 haloalkyl, a benzyl, 4- methoxybenzyl, pentafluorophenyl or a group of formula j? ;
in the presence of a catalyst and, if L1 is a hydroxyl group, in the presence of a condensing agent.
13. A process according to claim 12, characterised in that R1 is a hydrogen atom, and that the process is completed by a further step according to the following reaction scheme :
(Ib) (Viπ) (Ia) in which : - R2, R3, X, n, W, R4, R5, R6 and Het are as defined in claim; - L2 is a leaving group chosen as being a halogen atom, a 4- methyl phenylsulfonyloxy or a methylsulfonyloxy; and
- R1 a is a Ci-Cό-alkyl group or a Cβ-Cy-cycloalkyl; comprising the reaction of a compound of general formula (Ib) with a compound of general formula (VIII) to provide a compound of general formula (Ia).
14. A compound of general formula (II)
15. A fungicide composition comprising an effective amount of a compound according to any of the claims 1 to 11 and an agriculturally acceptable support.
16. A method for preventively or curatively combating the phytopathogenic fungi of crops, characterised in that an effective and non-phytotoxic amount of a composition according to claim 15 is applied to the plant seeds or to the plant leaves and/or to the fruits of the plants or to the soil in which the plants are growing or in which it is desired to grow them.
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008534004A JP5154424B2 (en) | 2005-10-05 | 2006-10-04 | Novel N-alkyl-heterocyclylcarboxamide derivatives |
| PL06806950T PL1937637T3 (en) | 2005-10-05 | 2006-10-04 | New n-alkyl-heterocyclyl carboxamide derivatives |
| ES06806950T ES2410879T3 (en) | 2005-10-05 | 2006-10-04 | New N-alkyl-heterocyclylcarboxamide derivatives |
| EP06806950A EP1937637B1 (en) | 2005-10-05 | 2006-10-04 | New n-alkyl-heterocyclyl carboxamide derivatives |
| US12/083,123 US8148538B2 (en) | 2005-10-05 | 2006-10-04 | N-alkyl-heterocyclyl carboxamide derivatives |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05356179.1 | 2005-10-05 | ||
| EP05356179A EP1772449A1 (en) | 2005-10-05 | 2005-10-05 | New N-alkyl-heterocyclyl carboxamide derivatives |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2007039615A1 true WO2007039615A1 (en) | 2007-04-12 |
Family
ID=36046823
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2006/067013 WO2007039615A1 (en) | 2005-10-05 | 2006-10-04 | New n-alkyl-heterocyclyl carboxamide derivatives |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US8148538B2 (en) |
| EP (2) | EP1772449A1 (en) |
| JP (1) | JP5154424B2 (en) |
| ES (1) | ES2410879T3 (en) |
| PL (1) | PL1937637T3 (en) |
| WO (1) | WO2007039615A1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011101256A1 (en) | 2010-02-18 | 2011-08-25 | Syngenta Participations Ag | Pyrazole microbiocides |
| CN102933590A (en) * | 2010-06-03 | 2013-02-13 | 拜尔农科股份公司 | Fungicide n-[(trisubstitutedsilyl)methyl]-carboxamide derivatives |
| US20190014779A1 (en) * | 2015-12-30 | 2019-01-17 | E. I. Du Pont De Nemours And Company | Nematocidal heterocyclic amides |
| EP4389739A1 (en) | 2022-12-21 | 2024-06-26 | Corteva Agriscience LLC | Molecule having pesticidal utility and processes related thereto |
| WO2024137594A1 (en) | 2022-12-21 | 2024-06-27 | Corteva Agriscience Llc | Molecule having pesticidal utility and processes related thereto |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BRPI1004930B1 (en) * | 2009-01-28 | 2017-10-17 | Bayer Intellectual Property Gmbh | Compounds, fungicidal composition and method for controlling phytopathogenic fungi of crops. |
| US8999956B2 (en) * | 2010-06-03 | 2015-04-07 | Bayer Intellectual Property Gmbh | N-[(het)arylalkyl)] pyrazole(thio)carboxamides and their heterosubstituted analogues |
| WO2021122975A1 (en) * | 2019-12-20 | 2021-06-24 | Bayer Aktiengesellschaft | Substituted thiophene carboxamides and derivatives thereof as microbicides |
| KR20220119651A (en) * | 2019-12-20 | 2022-08-30 | 바이엘 악티엔게젤샤프트 | Substituted thiophene carboxamides, thiophene carboxylic acids and derivatives thereof |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1387652A (en) * | 1972-05-10 | 1975-03-19 | Shell Int Research | Process for preparing furan carboxamides |
| US5240951A (en) * | 1990-09-20 | 1993-08-31 | Mitsui Toatsu Chemicals, Incorporated | Isothiazolecarboxylic acid derivatives, rice blast control agents containing the same as active ingredients, and rice blast control method applying the control agents |
| US5451702A (en) * | 1993-04-26 | 1995-09-19 | Monsanto Company | Process for preparing substituted aromatic amines |
| WO1996029871A2 (en) * | 1995-03-31 | 1996-10-03 | Nihon Nohyaku Co., Ltd. | An agricultural and horticultural disease controller and a method for controlling the diseases |
| WO1996038419A1 (en) * | 1995-05-31 | 1996-12-05 | Nissan Chemical Industries, Ltd. | 5-pyrazolecarboxamide derivatives and plant disease control agent |
| JPH09176124A (en) * | 1995-12-25 | 1997-07-08 | Nissan Chem Ind Ltd | Pyrazoline derivative and plant blight controlling agent |
| JPH09188662A (en) * | 1996-01-10 | 1997-07-22 | Sumitomo Chem Co Ltd | Method for producing sulfonic acid amide compound |
| WO2003013517A1 (en) * | 2001-08-06 | 2003-02-20 | Pharmacia Italia S.P.A. | Aminoisoxazole derivatives active as kinase inhibitors |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3809053A1 (en) * | 1988-03-18 | 1989-09-28 | Bayer Ag | SUBSTITUTED 5-AMINO-4H-1,2,4-TRIAZOL-3-YL-CARBONIC ACID AMIDES, METHODS FOR THE PRODUCTION THEREOF AND THEIR USE AS HERBICIDES |
| JP2530071B2 (en) * | 1990-09-20 | 1996-09-04 | 三井東圧化学株式会社 | Isothiazolecarboxylic acid derivatives and rice blast control agents containing these as active ingredients |
| DE4124150A1 (en) * | 1991-07-20 | 1993-01-21 | Bayer Ag | SUBSTITUTED TRIAZOLES |
| JPH09176125A (en) * | 1995-05-31 | 1997-07-08 | Nissan Chem Ind Ltd | 5-pyrazolecarboxylic acid amide derivative and plant blight controlling agent |
| JP2001097959A (en) * | 1999-10-01 | 2001-04-10 | Hokko Chem Ind Co Ltd | Isoxazole derivatives and plant disease control agents for agricultural and horticultural use |
| AU2002363250A1 (en) * | 2001-11-01 | 2003-05-12 | Icagen, Inc. | Pyrazole-amides and-sulfonamides |
| US6825209B2 (en) * | 2002-04-15 | 2004-11-30 | Research Triangle Institute | Compounds having unique CB1 receptor binding selectivity and methods for their production and use |
| JP2008525401A (en) * | 2004-12-23 | 2008-07-17 | アストラゼネカ アクチボラグ | Remedy |
-
2005
- 2005-10-05 EP EP05356179A patent/EP1772449A1/en not_active Withdrawn
-
2006
- 2006-10-04 WO PCT/EP2006/067013 patent/WO2007039615A1/en active Application Filing
- 2006-10-04 JP JP2008534004A patent/JP5154424B2/en not_active Expired - Fee Related
- 2006-10-04 EP EP06806950A patent/EP1937637B1/en not_active Not-in-force
- 2006-10-04 US US12/083,123 patent/US8148538B2/en not_active Expired - Fee Related
- 2006-10-04 PL PL06806950T patent/PL1937637T3/en unknown
- 2006-10-04 ES ES06806950T patent/ES2410879T3/en active Active
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1387652A (en) * | 1972-05-10 | 1975-03-19 | Shell Int Research | Process for preparing furan carboxamides |
| US5240951A (en) * | 1990-09-20 | 1993-08-31 | Mitsui Toatsu Chemicals, Incorporated | Isothiazolecarboxylic acid derivatives, rice blast control agents containing the same as active ingredients, and rice blast control method applying the control agents |
| US5451702A (en) * | 1993-04-26 | 1995-09-19 | Monsanto Company | Process for preparing substituted aromatic amines |
| WO1996029871A2 (en) * | 1995-03-31 | 1996-10-03 | Nihon Nohyaku Co., Ltd. | An agricultural and horticultural disease controller and a method for controlling the diseases |
| WO1996038419A1 (en) * | 1995-05-31 | 1996-12-05 | Nissan Chemical Industries, Ltd. | 5-pyrazolecarboxamide derivatives and plant disease control agent |
| JPH09176124A (en) * | 1995-12-25 | 1997-07-08 | Nissan Chem Ind Ltd | Pyrazoline derivative and plant blight controlling agent |
| JPH09188662A (en) * | 1996-01-10 | 1997-07-22 | Sumitomo Chem Co Ltd | Method for producing sulfonic acid amide compound |
| WO2003013517A1 (en) * | 2001-08-06 | 2003-02-20 | Pharmacia Italia S.P.A. | Aminoisoxazole derivatives active as kinase inhibitors |
Non-Patent Citations (4)
| Title |
|---|
| CANTRILL A A ET AL: "Preparation and Ring-Opening Reactions of N-Diphenylphosphinyl Aziridines", TETRAHEDRON, ELSEVIER SCIENCE PUBLISHERS, AMSTERDAM, NL, vol. 54, no. 10, 5 March 1998 (1998-03-05), pages 2181 - 2208, XP004109555, ISSN: 0040-4020 * |
| DATABASE CAPLUS [online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 1997, XP002375266, retrieved from STN Database accession no. 1363 * |
| PATENT ABSTRACTS OF JAPAN vol. 1997, no. 11 28 November 1997 (1997-11-28) * |
| THOMAS ET AL: "Synthesis of long-chain amide analogs of the cannabinoid CB1 receptor antagonist N-(piperidinyl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)- 4-methyl-1H-pyrazole-3-carboxamide (SR141716) with unique binding selectivities and pharmacological activities", BIOORGANIC & MEDICINAL CHEMISTRY, ELSEVIER SCIENCE LTD, GB, vol. 13, no. 18, 15 September 2005 (2005-09-15), pages 5463 - 5474, XP005021048, ISSN: 0968-0896 * |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011101256A1 (en) | 2010-02-18 | 2011-08-25 | Syngenta Participations Ag | Pyrazole microbiocides |
| CN102762540A (en) * | 2010-02-18 | 2012-10-31 | 先正达参股股份有限公司 | Pyrazole microbiocides |
| US8536215B2 (en) | 2010-02-18 | 2013-09-17 | Syngenta Crop Protection Llc | Pyrazole microbiocides |
| CN102762540B (en) * | 2010-02-18 | 2014-12-17 | 先正达参股股份有限公司 | Pyrazole microbiocides |
| CN102933590A (en) * | 2010-06-03 | 2013-02-13 | 拜尔农科股份公司 | Fungicide n-[(trisubstitutedsilyl)methyl]-carboxamide derivatives |
| CN102933590B (en) * | 2010-06-03 | 2016-05-18 | 拜尔农科股份公司 | The trisubstituted silicyl of antifungal N-[() methyl]-carboxamide derivative |
| US20190014779A1 (en) * | 2015-12-30 | 2019-01-17 | E. I. Du Pont De Nemours And Company | Nematocidal heterocyclic amides |
| US10820590B2 (en) * | 2015-12-30 | 2020-11-03 | E I Du Pont De Nemours And Company | Nematocidal heterocyclic amides |
| EP4389739A1 (en) | 2022-12-21 | 2024-06-26 | Corteva Agriscience LLC | Molecule having pesticidal utility and processes related thereto |
| WO2024137594A1 (en) | 2022-12-21 | 2024-06-27 | Corteva Agriscience Llc | Molecule having pesticidal utility and processes related thereto |
Also Published As
| Publication number | Publication date |
|---|---|
| PL1937637T3 (en) | 2013-08-30 |
| US20100197633A1 (en) | 2010-08-05 |
| EP1937637A1 (en) | 2008-07-02 |
| JP2009511447A (en) | 2009-03-19 |
| JP5154424B2 (en) | 2013-02-27 |
| EP1937637B1 (en) | 2013-04-03 |
| EP1772449A1 (en) | 2007-04-11 |
| ES2410879T3 (en) | 2013-07-03 |
| US8148538B2 (en) | 2012-04-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1960379B1 (en) | N-(1-alkyl-2-phenylethyl)-carboxamide derivatives and use thereof as fungicides | |
| EP1954674B1 (en) | New n-phenethylcarboxamide derivatives | |
| EP2125740B1 (en) | Fungicide n-(3-phenylpropyl)carboxamide derivatives | |
| EP1881978B1 (en) | Fungicide 2-pyridyl-methylene-carboxamide derivatives | |
| EP1879878B1 (en) | Heterocyclylethylheterocyclylcarboxamide derivatives as fungicides | |
| WO2006122955A1 (en) | New 2-pyridinylcycloalkylcarboxamide derivatives as fungicides. | |
| US8148538B2 (en) | N-alkyl-heterocyclyl carboxamide derivatives | |
| US20090105308A1 (en) | Benzoheterocylethylcarboxamide derivatives | |
| US20080096932A1 (en) | 3-Pyridinylethylcarboxamide Derivatives as Fungicides | |
| WO2006108792A1 (en) | New heterocyclylethylbenzamide derivatives | |
| WO2007006734A1 (en) | New benzoheterocyclylethylbenzamide derivatives | |
| WO2006008193A1 (en) | 4-pyridinylethylcarboxamide derivatives useful as fungicides | |
| WO2008081011A1 (en) | N-methyl carboxamide derivatives useful as fungicides | |
| WO2008081017A1 (en) | New n-alkynylcarboxamide derivatives | |
| WO2008003741A1 (en) | N- [ (pyridin- 2 -yl) methoxy]heterocyclyl carboxamide derivatives and related compounds as fungicides |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2006806950 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12083123 Country of ref document: US Ref document number: 2008534004 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWP | Wipo information: published in national office |
Ref document number: 2006806950 Country of ref document: EP |