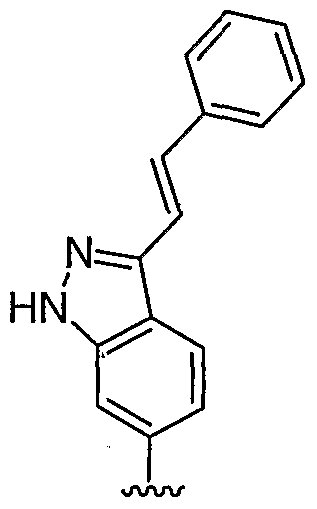
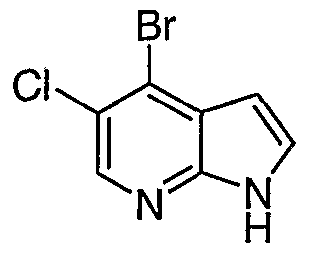
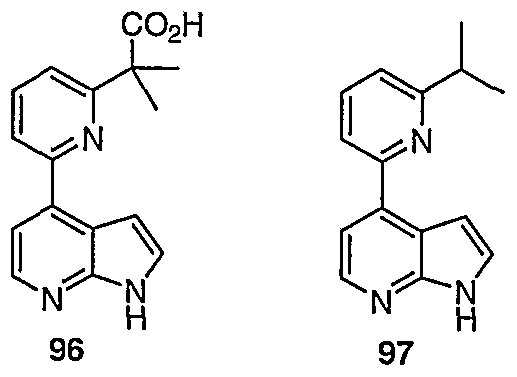
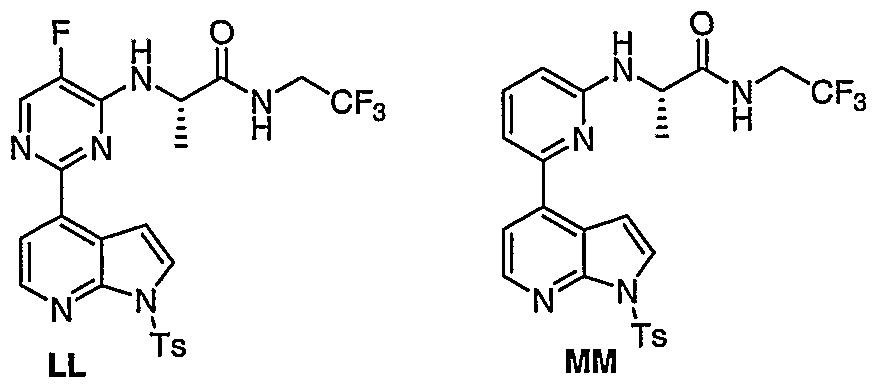
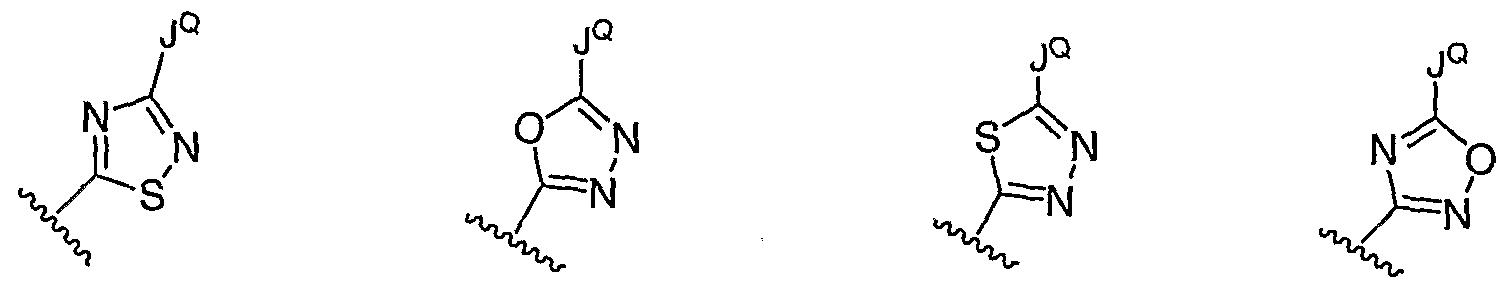WO2006127587A1 - Pyrrolopyridines useful as inhibitors of protein kinase - Google Patents
Pyrrolopyridines useful as inhibitors of protein kinase Download PDFInfo
- Publication number
- WO2006127587A1 WO2006127587A1 PCT/US2006/019711 US2006019711W WO2006127587A1 WO 2006127587 A1 WO2006127587 A1 WO 2006127587A1 US 2006019711 W US2006019711 W US 2006019711W WO 2006127587 A1 WO2006127587 A1 WO 2006127587A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound according
- aliphatic
- optionally substituted
- membered
- halogen
- Prior art date
Links
- 239000003112 inhibitor Substances 0.000 title abstract description 11
- 102000001253 Protein Kinase Human genes 0.000 title abstract description 8
- 108060006633 protein kinase Proteins 0.000 title abstract description 8
- 150000005255 pyrrolopyridines Chemical class 0.000 title description 2
- 150000001875 compounds Chemical class 0.000 claims abstract description 349
- 239000000203 mixture Substances 0.000 claims abstract description 169
- 238000000034 method Methods 0.000 claims abstract description 122
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 70
- 201000010099 disease Diseases 0.000 claims abstract description 39
- 239000011435 rock Substances 0.000 claims abstract description 25
- 108091000080 Phosphotransferase Proteins 0.000 claims abstract description 19
- 102000020233 phosphotransferase Human genes 0.000 claims abstract description 19
- 102000042838 JAK family Human genes 0.000 claims abstract description 11
- 108091082332 JAK family Proteins 0.000 claims abstract description 11
- -1 NCH3 Chemical group 0.000 claims description 123
- 125000001931 aliphatic group Chemical group 0.000 claims description 119
- 229910052736 halogen Inorganic materials 0.000 claims description 78
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 71
- 125000001424 substituent group Chemical group 0.000 claims description 66
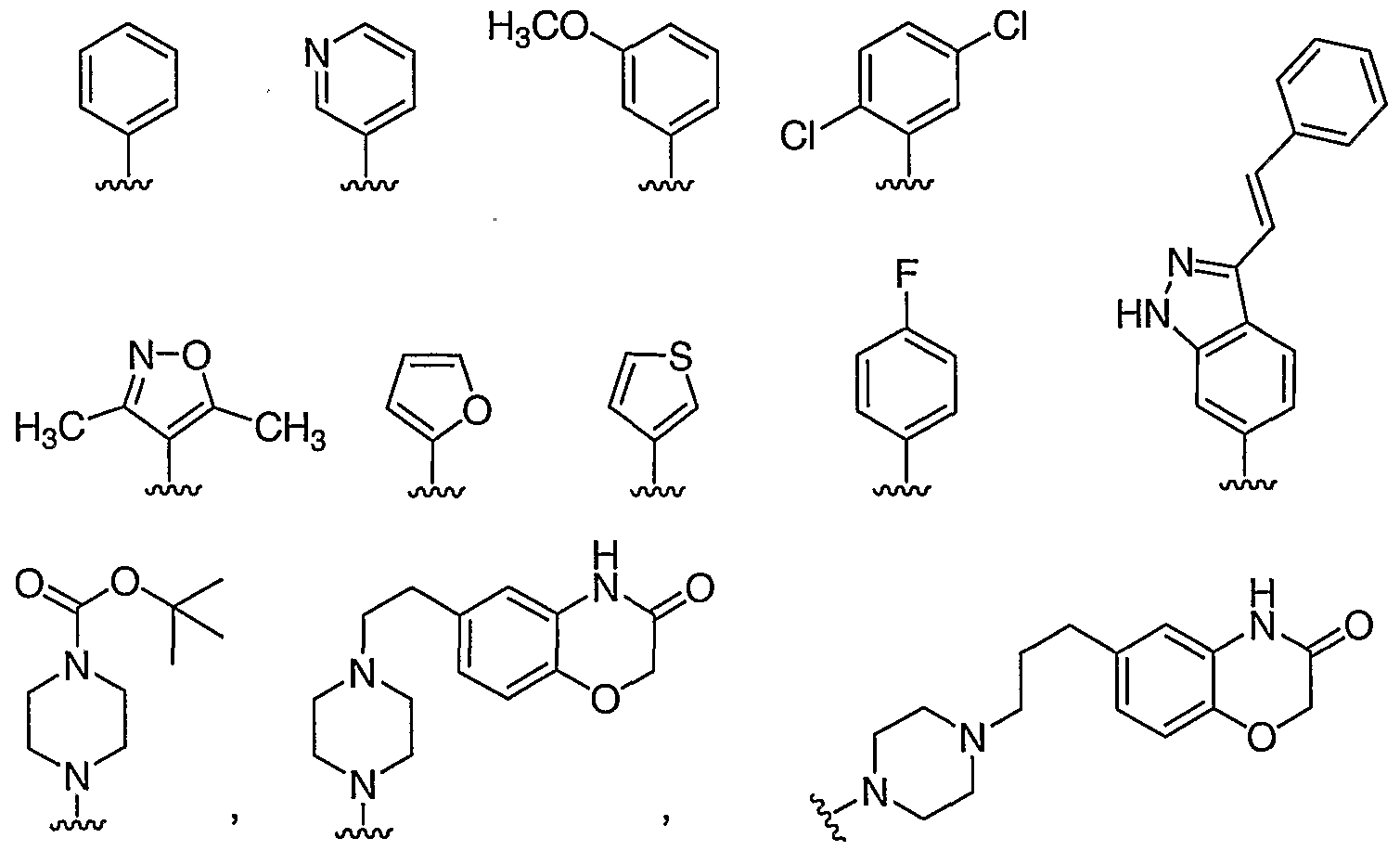
- 125000000339 4-pyridyl group Chemical group N1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 claims description 65
- 125000000623 heterocyclic group Chemical group 0.000 claims description 65
- 239000007787 solid Substances 0.000 claims description 64
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 claims description 63
- 229910052757 nitrogen Inorganic materials 0.000 claims description 55
- 150000002367 halogens Chemical class 0.000 claims description 54
- 239000003795 chemical substances by application Substances 0.000 claims description 52
- 125000004429 atom Chemical group 0.000 claims description 37
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 32
- 125000003118 aryl group Chemical group 0.000 claims description 30
- 208000035475 disorder Diseases 0.000 claims description 30
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 claims description 29
- 125000005843 halogen group Chemical group 0.000 claims description 29
- 229910052739 hydrogen Inorganic materials 0.000 claims description 29
- 229920006395 saturated elastomer Polymers 0.000 claims description 29
- 229910052760 oxygen Inorganic materials 0.000 claims description 28
- 239000003814 drug Substances 0.000 claims description 27
- 150000003839 salts Chemical class 0.000 claims description 27
- 229910052801 chlorine Inorganic materials 0.000 claims description 22
- 125000001072 heteroaryl group Chemical group 0.000 claims description 22
- 201000006417 multiple sclerosis Diseases 0.000 claims description 22
- 229940124597 therapeutic agent Drugs 0.000 claims description 22
- 125000001313 C5-C10 heteroaryl group Chemical group 0.000 claims description 20
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 19
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 18
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 18
- 125000005842 heteroatom Chemical group 0.000 claims description 18
- 125000002950 monocyclic group Chemical group 0.000 claims description 18
- 239000001301 oxygen Chemical group 0.000 claims description 18
- 229910052717 sulfur Chemical group 0.000 claims description 18
- 239000011593 sulfur Chemical group 0.000 claims description 18
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 claims description 17
- 239000002552 dosage form Substances 0.000 claims description 16
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 claims description 15
- 208000006673 asthma Diseases 0.000 claims description 15
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 15
- 230000000694 effects Effects 0.000 claims description 15
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 claims description 14
- 208000010228 Erectile Dysfunction Diseases 0.000 claims description 13
- 201000001881 impotence Diseases 0.000 claims description 13
- 206010007572 Cardiac hypertrophy Diseases 0.000 claims description 12
- 206010020772 Hypertension Diseases 0.000 claims description 12
- 125000002619 bicyclic group Chemical group 0.000 claims description 11
- 239000012472 biological sample Substances 0.000 claims description 11
- 206010012601 diabetes mellitus Diseases 0.000 claims description 11
- 208000028867 ischemia Diseases 0.000 claims description 11
- 230000010410 reperfusion Effects 0.000 claims description 11
- 208000023275 Autoimmune disease Diseases 0.000 claims description 10
- 208000010412 Glaucoma Diseases 0.000 claims description 10
- 229910052799 carbon Inorganic materials 0.000 claims description 10
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 10
- 230000001404 mediated effect Effects 0.000 claims description 10
- 208000024827 Alzheimer disease Diseases 0.000 claims description 9
- 206010003225 Arteriospasm coronary Diseases 0.000 claims description 9
- 201000001320 Atherosclerosis Diseases 0.000 claims description 9
- 125000000041 C6-C10 aryl group Chemical group 0.000 claims description 9
- 208000006029 Cardiomegaly Diseases 0.000 claims description 9
- 208000024172 Cardiovascular disease Diseases 0.000 claims description 9
- 208000003890 Coronary Vasospasm Diseases 0.000 claims description 9
- 201000011634 coronary artery vasospasm Diseases 0.000 claims description 9
- 125000004122 cyclic group Chemical group 0.000 claims description 9
- 239000003937 drug carrier Substances 0.000 claims description 9
- 208000001286 intracranial vasospasm Diseases 0.000 claims description 9
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 9
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 claims description 9
- 239000003981 vehicle Substances 0.000 claims description 9
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 8
- 208000011231 Crohn disease Diseases 0.000 claims description 8
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 8
- 230000002401 inhibitory effect Effects 0.000 claims description 8
- 206010002383 Angina Pectoris Diseases 0.000 claims description 7
- 206010020751 Hypersensitivity Diseases 0.000 claims description 7
- 208000029462 Immunodeficiency disease Diseases 0.000 claims description 7
- 208000014767 Myeloproliferative disease Diseases 0.000 claims description 7
- 108010025020 Nerve Growth Factor Proteins 0.000 claims description 7
- 102000007072 Nerve Growth Factors Human genes 0.000 claims description 7
- 206010052779 Transplant rejections Diseases 0.000 claims description 7
- 229940121363 anti-inflammatory agent Drugs 0.000 claims description 7
- 239000002260 anti-inflammatory agent Substances 0.000 claims description 7
- 230000001028 anti-proliverative effect Effects 0.000 claims description 7
- UKJLNMAFNRKWGR-UHFFFAOYSA-N cyclohexatrienamine Chemical group NC1=CC=C=C[CH]1 UKJLNMAFNRKWGR-UHFFFAOYSA-N 0.000 claims description 7
- 229910052731 fluorine Inorganic materials 0.000 claims description 7
- 239000001257 hydrogen Substances 0.000 claims description 7
- 230000002519 immonomodulatory effect Effects 0.000 claims description 7
- 229940125721 immunosuppressive agent Drugs 0.000 claims description 7
- 239000003018 immunosuppressive agent Substances 0.000 claims description 7
- 239000003900 neurotrophic factor Substances 0.000 claims description 7
- 210000000056 organ Anatomy 0.000 claims description 7
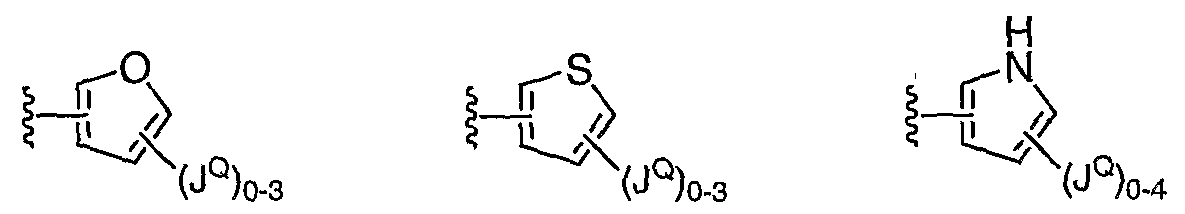
- 125000001544 thienyl group Chemical group 0.000 claims description 7
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 6
- 208000020084 Bone disease Diseases 0.000 claims description 6
- 206010028980 Neoplasm Diseases 0.000 claims description 6
- 208000003782 Raynaud disease Diseases 0.000 claims description 6
- 208000012322 Raynaud phenomenon Diseases 0.000 claims description 6
- 208000006011 Stroke Diseases 0.000 claims description 6
- 208000027418 Wounds and injury Diseases 0.000 claims description 6
- 239000002671 adjuvant Substances 0.000 claims description 6
- 201000011510 cancer Diseases 0.000 claims description 6
- 230000004663 cell proliferation Effects 0.000 claims description 6
- 230000000973 chemotherapeutic effect Effects 0.000 claims description 6
- 230000006378 damage Effects 0.000 claims description 6
- 208000014674 injury Diseases 0.000 claims description 6
- 230000014511 neuron projection development Effects 0.000 claims description 6
- 210000004509 vascular smooth muscle cell Anatomy 0.000 claims description 6
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 claims description 5
- 208000030507 AIDS Diseases 0.000 claims description 5
- 208000019838 Blood disease Diseases 0.000 claims description 5
- 206010048554 Endothelial dysfunction Diseases 0.000 claims description 5
- 206010016654 Fibrosis Diseases 0.000 claims description 5
- 208000002250 Hematologic Neoplasms Diseases 0.000 claims description 5
- 206010025323 Lymphomas Diseases 0.000 claims description 5
- 208000018737 Parkinson disease Diseases 0.000 claims description 5
- 125000003545 alkoxy group Chemical group 0.000 claims description 5
- 239000003443 antiviral agent Substances 0.000 claims description 5
- 230000008694 endothelial dysfunction Effects 0.000 claims description 5
- 230000004761 fibrosis Effects 0.000 claims description 5
- 208000014951 hematologic disease Diseases 0.000 claims description 5
- 208000018706 hematopoietic system disease Diseases 0.000 claims description 5
- 208000032839 leukemia Diseases 0.000 claims description 5
- 208000019423 liver disease Diseases 0.000 claims description 5
- 208000015122 neurodegenerative disease Diseases 0.000 claims description 5
- 125000000636 p-nitrophenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)[N+]([O-])=O 0.000 claims description 5
- 230000002062 proliferating effect Effects 0.000 claims description 5
- 125000003226 pyrazolyl group Chemical group 0.000 claims description 5
- 125000004076 pyridyl group Chemical group 0.000 claims description 5
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 5
- 201000000980 schizophrenia Diseases 0.000 claims description 5
- 125000004211 3,5-difluorophenyl group Chemical group [H]C1=C(F)C([H])=C(*)C([H])=C1F 0.000 claims description 4
- 201000004384 Alopecia Diseases 0.000 claims description 4
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 claims description 4
- 208000020446 Cardiac disease Diseases 0.000 claims description 4
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 claims description 4
- 206010012289 Dementia Diseases 0.000 claims description 4
- 208000023105 Huntington disease Diseases 0.000 claims description 4
- 206010020880 Hypertrophy Diseases 0.000 claims description 4
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 claims description 4
- 201000007224 Myeloproliferative neoplasm Diseases 0.000 claims description 4
- 230000007815 allergy Effects 0.000 claims description 4
- 125000001246 bromo group Chemical group Br* 0.000 claims description 4
- 210000004413 cardiac myocyte Anatomy 0.000 claims description 4
- 206010009887 colitis Diseases 0.000 claims description 4
- 230000001066 destructive effect Effects 0.000 claims description 4
- 125000003754 ethoxycarbonyl group Chemical group C(=O)(OCC)* 0.000 claims description 4
- 230000003676 hair loss Effects 0.000 claims description 4
- 208000019622 heart disease Diseases 0.000 claims description 4
- 208000027866 inflammatory disease Diseases 0.000 claims description 4
- 208000030603 inherited susceptibility to asthma Diseases 0.000 claims description 4
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 claims description 4
- 239000008194 pharmaceutical composition Substances 0.000 claims description 4
- 125000003373 pyrazinyl group Chemical group 0.000 claims description 4
- 125000002098 pyridazinyl group Chemical group 0.000 claims description 4
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 4
- 125000000168 pyrrolyl group Chemical group 0.000 claims description 4
- 230000003612 virological effect Effects 0.000 claims description 4
- 206010004446 Benign prostatic hyperplasia Diseases 0.000 claims description 3
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 claims description 3
- 208000032027 Essential Thrombocythemia Diseases 0.000 claims description 3
- 208000009329 Graft vs Host Disease Diseases 0.000 claims description 3
- 206010048643 Hypereosinophilic syndrome Diseases 0.000 claims description 3
- 208000035268 Mast Cell Activation disease Diseases 0.000 claims description 3
- 206010028561 Myeloid metaplasia Diseases 0.000 claims description 3
- 208000033833 Myelomonocytic Chronic Leukemia Diseases 0.000 claims description 3
- 208000001132 Osteoporosis Diseases 0.000 claims description 3
- 208000006399 Premature Obstetric Labor Diseases 0.000 claims description 3
- 208000004403 Prostatic Hyperplasia Diseases 0.000 claims description 3
- 208000017442 Retinal disease Diseases 0.000 claims description 3
- 206010038923 Retinopathy Diseases 0.000 claims description 3
- 208000017733 acquired polycythemia vera Diseases 0.000 claims description 3
- 208000026935 allergic disease Diseases 0.000 claims description 3
- 230000000172 allergic effect Effects 0.000 claims description 3
- 208000010216 atopic IgE responsiveness Diseases 0.000 claims description 3
- 229910052794 bromium Inorganic materials 0.000 claims description 3
- 208000021668 chronic eosinophilic leukemia Diseases 0.000 claims description 3
- 201000010902 chronic myelomonocytic leukemia Diseases 0.000 claims description 3
- 230000008602 contraction Effects 0.000 claims description 3
- 125000002541 furyl group Chemical group 0.000 claims description 3
- 208000024908 graft versus host disease Diseases 0.000 claims description 3
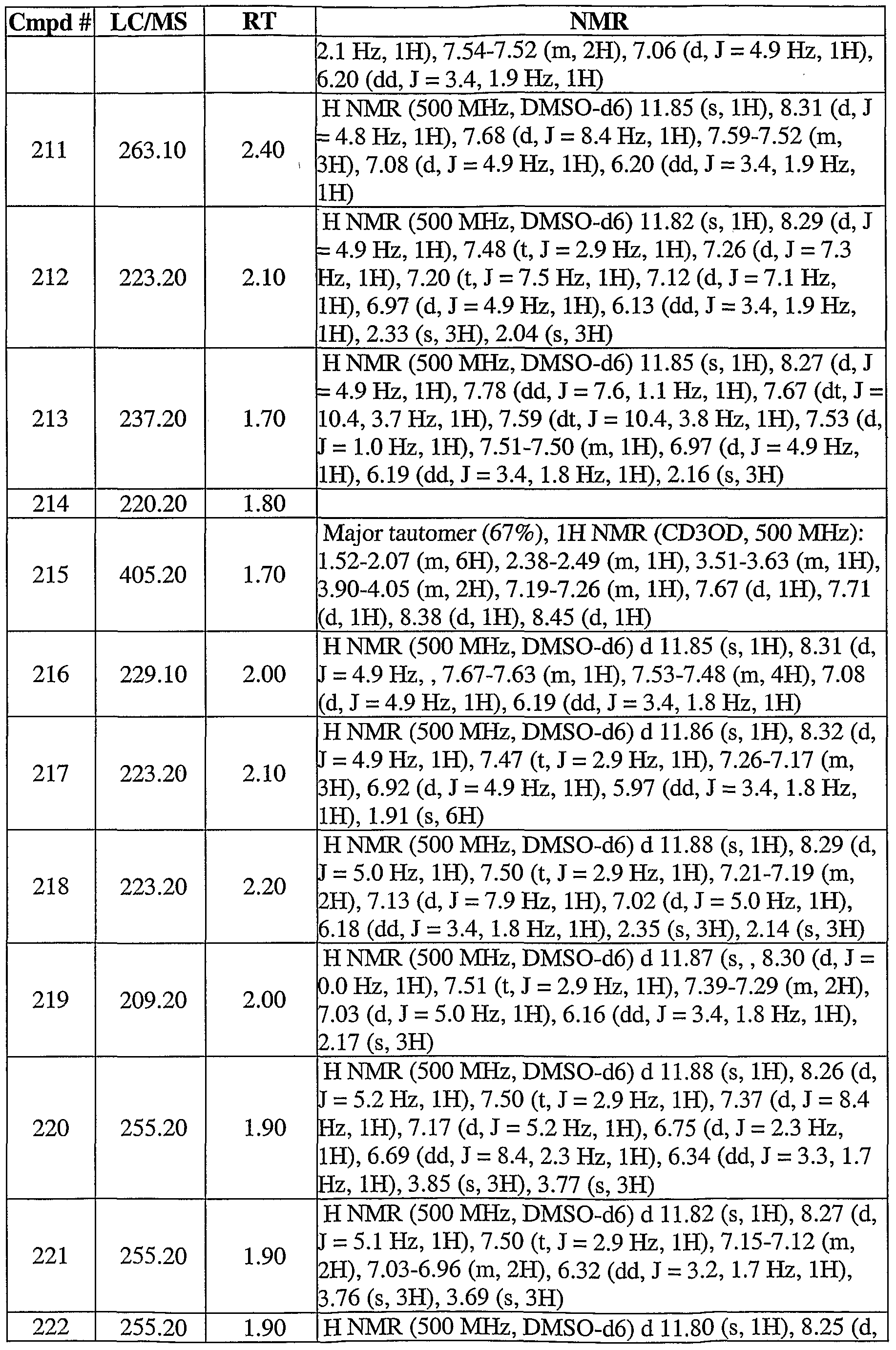
- 206010028537 myelofibrosis Diseases 0.000 claims description 3
- 230000003836 peripheral circulation Effects 0.000 claims description 3
- 208000037244 polycythemia vera Diseases 0.000 claims description 3
- 208000003476 primary myelofibrosis Diseases 0.000 claims description 3
- 208000037803 restenosis Diseases 0.000 claims description 3
- 230000009897 systematic effect Effects 0.000 claims description 3
- 125000004306 triazinyl group Chemical group 0.000 claims description 3
- 201000003883 Cystic fibrosis Diseases 0.000 claims description 2
- 206010019842 Hepatomegaly Diseases 0.000 claims description 2
- 206010061218 Inflammation Diseases 0.000 claims description 2
- 208000005107 Premature Birth Diseases 0.000 claims description 2
- 206010036590 Premature baby Diseases 0.000 claims description 2
- 201000004681 Psoriasis Diseases 0.000 claims description 2
- 208000028017 Psychotic disease Diseases 0.000 claims description 2
- 229910006074 SO2NH2 Inorganic materials 0.000 claims description 2
- 206010047163 Vasospasm Diseases 0.000 claims description 2
- 239000000164 antipsychotic agent Substances 0.000 claims description 2
- 125000002618 bicyclic heterocycle group Chemical group 0.000 claims description 2
- CBOIHMRHGLHBPB-UHFFFAOYSA-N hydroxymethyl Chemical compound O[CH2] CBOIHMRHGLHBPB-UHFFFAOYSA-N 0.000 claims description 2
- 230000004054 inflammatory process Effects 0.000 claims description 2
- 125000004043 oxo group Chemical group O=* 0.000 claims 6
- 125000004767 (C1-C4) haloalkoxy group Chemical group 0.000 claims 2
- 125000004172 4-methoxyphenyl group Chemical group [H]C1=C([H])C(OC([H])([H])[H])=C([H])C([H])=C1* 0.000 claims 2
- 125000000335 thiazolyl group Chemical group 0.000 claims 2
- 125000006583 (C1-C3) haloalkyl group Chemical group 0.000 claims 1
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims 1
- 238000011282 treatment Methods 0.000 abstract description 12
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 165
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 97
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 82
- 239000000243 solution Substances 0.000 description 74
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 72
- 235000019439 ethyl acetate Nutrition 0.000 description 64
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 58
- 238000006243 chemical reaction Methods 0.000 description 46
- 239000002904 solvent Substances 0.000 description 46
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 42
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 41
- 239000011541 reaction mixture Substances 0.000 description 41
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 39
- 230000015572 biosynthetic process Effects 0.000 description 37
- 229940093499 ethyl acetate Drugs 0.000 description 37
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 35
- 238000003786 synthesis reaction Methods 0.000 description 35
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 33
- 235000002639 sodium chloride Nutrition 0.000 description 33
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 31
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 30
- 229910052938 sodium sulfate Inorganic materials 0.000 description 30
- 239000007832 Na2SO4 Substances 0.000 description 26
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 25
- 239000000047 product Substances 0.000 description 20
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 19
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 19
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 18
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 18
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 18
- 239000012267 brine Substances 0.000 description 18
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 18
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 18
- 125000004093 cyano group Chemical group *C#N 0.000 description 17
- DTQVDTLACAAQTR-UHFFFAOYSA-N trifluoroacetic acid Substances OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 17
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 16
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical class [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 16
- 239000012044 organic layer Substances 0.000 description 16
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 15
- 239000003921 oil Substances 0.000 description 15
- 235000019198 oils Nutrition 0.000 description 15
- 239000000725 suspension Substances 0.000 description 15
- ICSNLGPSRYBMBD-UHFFFAOYSA-N 2-aminopyridine Chemical class NC1=CC=CC=N1 ICSNLGPSRYBMBD-UHFFFAOYSA-N 0.000 description 14
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 14
- 239000002253 acid Substances 0.000 description 13
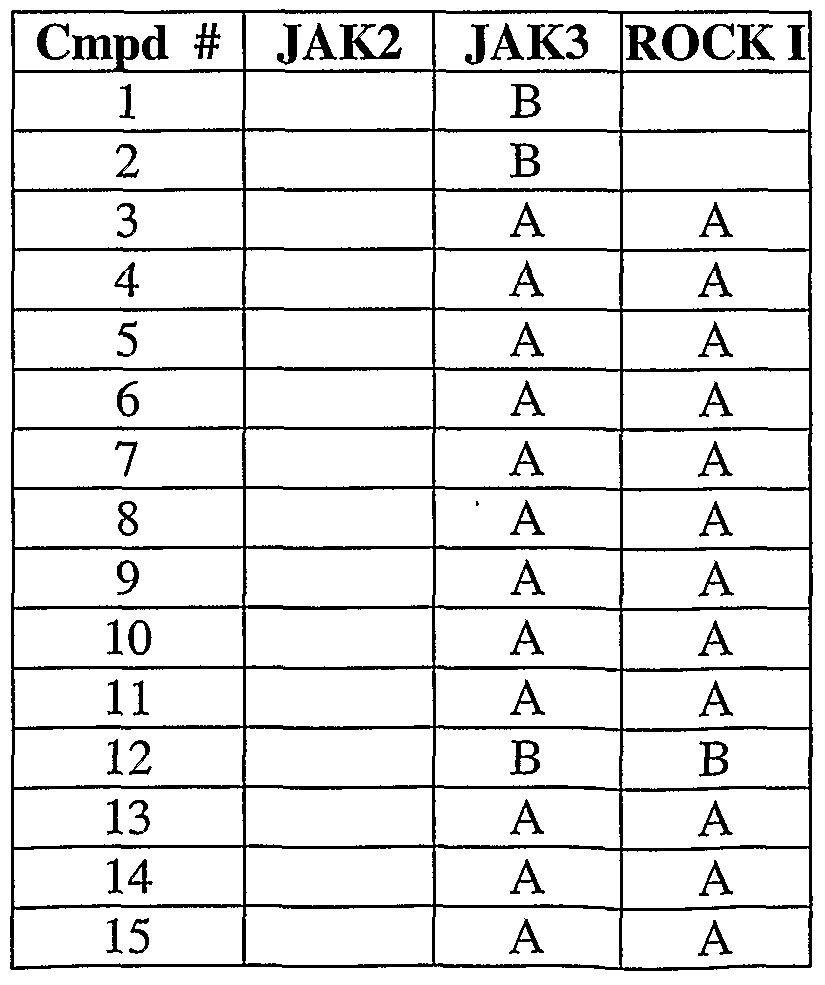
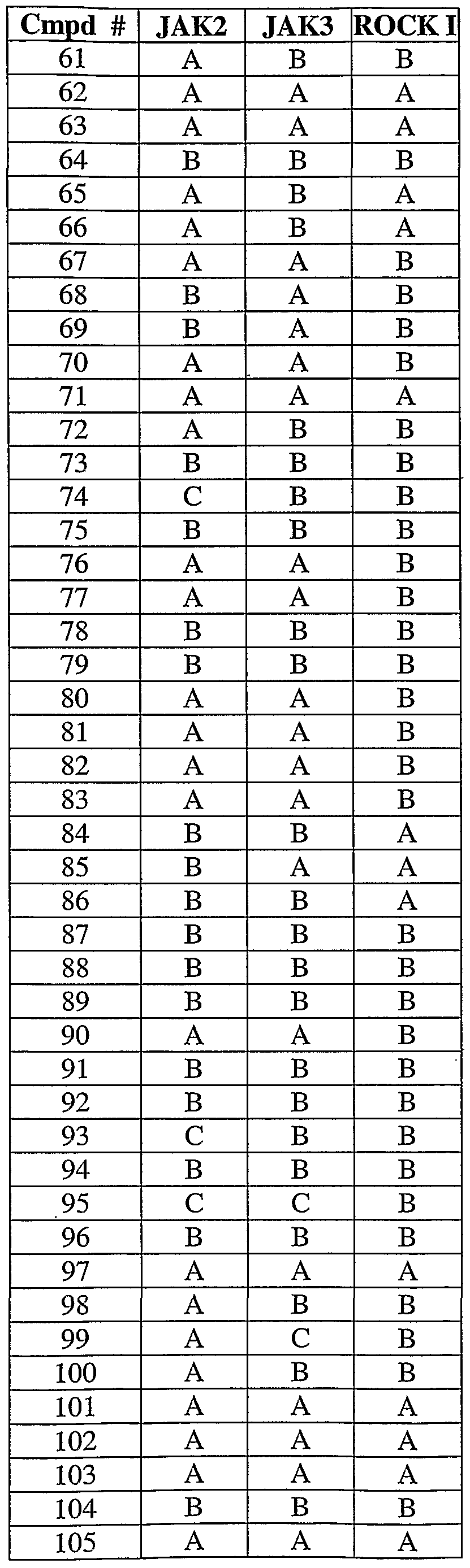
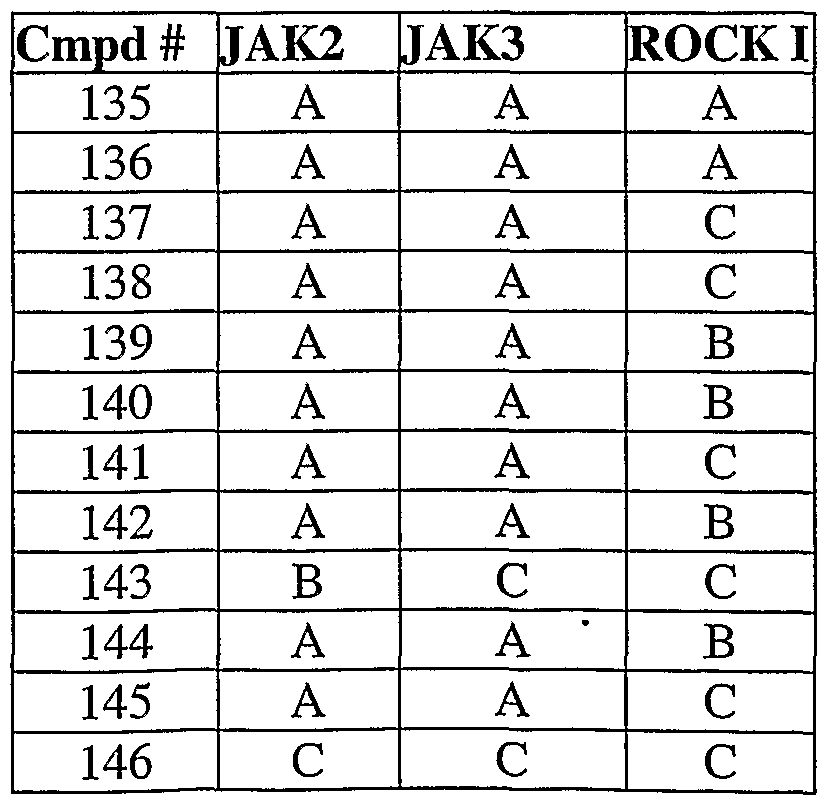
- 108010024121 Janus Kinases Proteins 0.000 description 12
- 102000015617 Janus Kinases Human genes 0.000 description 12
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- 150000001412 amines Chemical class 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- WYURNTSHIVDZCO-UHFFFAOYSA-N tetrahydrofuran Substances C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 12
- ZKHQWZAMYRWXGA-UHFFFAOYSA-N Adenosine triphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O ZKHQWZAMYRWXGA-UHFFFAOYSA-N 0.000 description 11
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 11
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 11
- 230000002829 reductive effect Effects 0.000 description 11
- 125000002088 tosyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C([H])([H])[H])S(*)(=O)=O 0.000 description 11
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 10
- 238000003556 assay Methods 0.000 description 10
- 238000000576 coating method Methods 0.000 description 10
- 238000002953 preparative HPLC Methods 0.000 description 10
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Substances CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 9
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 238000004440 column chromatography Methods 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 229910000027 potassium carbonate Inorganic materials 0.000 description 9
- 238000000746 purification Methods 0.000 description 9
- 229910000029 sodium carbonate Inorganic materials 0.000 description 9
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 8
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 8
- 238000004587 chromatography analysis Methods 0.000 description 8
- 238000003818 flash chromatography Methods 0.000 description 8
- 238000004128 high performance liquid chromatography Methods 0.000 description 8
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 8
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 8
- 229920001223 polyethylene glycol Polymers 0.000 description 8
- 239000000741 silica gel Substances 0.000 description 8
- 229910002027 silica gel Inorganic materials 0.000 description 8
- 229960001866 silicon dioxide Drugs 0.000 description 8
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 7
- NFHFRUOZVGFOOS-UHFFFAOYSA-N Pd(PPh3)4 Substances [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 7
- 125000000217 alkyl group Chemical group 0.000 description 7
- 239000002775 capsule Substances 0.000 description 7
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 7
- 239000008101 lactose Substances 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- KDLHZDBZIXYQEI-UHFFFAOYSA-N palladium Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 7
- 239000000546 pharmaceutical excipient Substances 0.000 description 7
- 238000004007 reversed phase HPLC Methods 0.000 description 7
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 7
- 239000003826 tablet Substances 0.000 description 7
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 6
- NUKYPUAOHBNCPY-UHFFFAOYSA-N 4-aminopyridine Chemical compound NC1=CC=NC=C1 NUKYPUAOHBNCPY-UHFFFAOYSA-N 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 101000934996 Homo sapiens Tyrosine-protein kinase JAK3 Proteins 0.000 description 6
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 6
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 6
- 102100025387 Tyrosine-protein kinase JAK3 Human genes 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- 239000006196 drop Substances 0.000 description 6
- 235000019441 ethanol Nutrition 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 231100000252 nontoxic Toxicity 0.000 description 6
- 230000003000 nontoxic effect Effects 0.000 description 6
- 229920000642 polymer Polymers 0.000 description 6
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- 239000011780 sodium chloride Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- HFUCVVCJXREYJQ-DFWYDOINSA-N (2s)-2-amino-n-(2,2,2-trifluoroethyl)propanamide;hydrochloride Chemical compound Cl.C[C@H](N)C(=O)NCC(F)(F)F HFUCVVCJXREYJQ-DFWYDOINSA-N 0.000 description 5
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 5
- 238000005160 1H NMR spectroscopy Methods 0.000 description 5
- PAQZWJGSJMLPMG-UHFFFAOYSA-N 2,4,6-tripropyl-1,3,5,2$l^{5},4$l^{5},6$l^{5}-trioxatriphosphinane 2,4,6-trioxide Chemical compound CCCP1(=O)OP(=O)(CCC)OP(=O)(CCC)O1 PAQZWJGSJMLPMG-UHFFFAOYSA-N 0.000 description 5
- YYROPELSRYBVMQ-UHFFFAOYSA-N 4-toluenesulfonyl chloride Chemical compound CC1=CC=C(S(Cl)(=O)=O)C=C1 YYROPELSRYBVMQ-UHFFFAOYSA-N 0.000 description 5
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 5
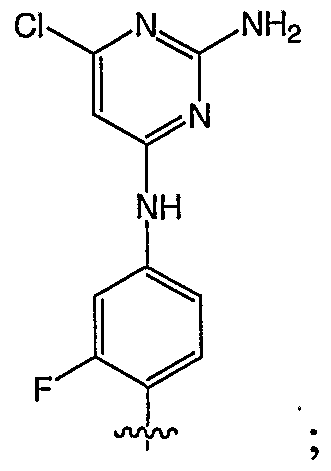
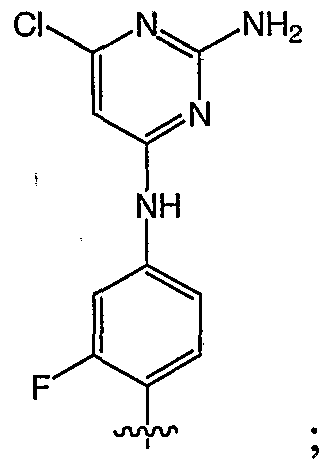
- 0 C*1C=CC(Nc2cc(F)c(C)cc2)=NC1N Chemical compound C*1C=CC(Nc2cc(F)c(C)cc2)=NC1N 0.000 description 5
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical class [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 5
- 102100033444 Tyrosine-protein kinase JAK2 Human genes 0.000 description 5
- 150000001408 amides Chemical class 0.000 description 5
- 239000000872 buffer Substances 0.000 description 5
- 230000008878 coupling Effects 0.000 description 5
- 238000010168 coupling process Methods 0.000 description 5
- 238000005859 coupling reaction Methods 0.000 description 5
- 239000010779 crude oil Substances 0.000 description 5
- 239000003085 diluting agent Substances 0.000 description 5
- 239000002270 dispersing agent Substances 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 239000003995 emulsifying agent Substances 0.000 description 5
- 150000002148 esters Chemical class 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 239000010410 layer Substances 0.000 description 5
- 239000000314 lubricant Substances 0.000 description 5
- 239000006187 pill Substances 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 239000003755 preservative agent Substances 0.000 description 5
- 239000000377 silicon dioxide Substances 0.000 description 5
- 239000007909 solid dosage form Substances 0.000 description 5
- 238000003756 stirring Methods 0.000 description 5
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 description 5
- 239000001993 wax Substances 0.000 description 5
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 4
- FIMYFEGKMOCQKT-UHFFFAOYSA-N 3,4-difluoro-2-(2-fluoro-4-iodoanilino)-n-(2-hydroxyethoxy)-5-[(3-oxooxazinan-2-yl)methyl]benzamide Chemical compound FC=1C(F)=C(NC=2C(=CC(I)=CC=2)F)C(C(=O)NOCCO)=CC=1CN1OCCCC1=O FIMYFEGKMOCQKT-UHFFFAOYSA-N 0.000 description 4
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 4
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 4
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 4
- 239000007995 HEPES buffer Substances 0.000 description 4
- 101000997832 Homo sapiens Tyrosine-protein kinase JAK2 Proteins 0.000 description 4
- 108010050904 Interferons Proteins 0.000 description 4
- 102000014150 Interferons Human genes 0.000 description 4
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 4
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- 229930006000 Sucrose Natural products 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 235000010443 alginic acid Nutrition 0.000 description 4
- 229920000615 alginic acid Polymers 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 4
- 150000001543 aryl boronic acids Chemical class 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 4
- 150000001805 chlorine compounds Chemical class 0.000 description 4
- JNGZXGGOCLZBFB-IVCQMTBJSA-N compound E Chemical compound N([C@@H](C)C(=O)N[C@@H]1C(N(C)C2=CC=CC=C2C(C=2C=CC=CC=2)=N1)=O)C(=O)CC1=CC(F)=CC(F)=C1 JNGZXGGOCLZBFB-IVCQMTBJSA-N 0.000 description 4
- 239000003246 corticosteroid Substances 0.000 description 4
- 229960001334 corticosteroids Drugs 0.000 description 4
- MLIREBYILWEBDM-UHFFFAOYSA-N cyanoacetic acid Chemical compound OC(=O)CC#N MLIREBYILWEBDM-UHFFFAOYSA-N 0.000 description 4
- 238000010511 deprotection reaction Methods 0.000 description 4
- 235000014113 dietary fatty acids Nutrition 0.000 description 4
- 239000000284 extract Substances 0.000 description 4
- 239000000194 fatty acid Substances 0.000 description 4
- 229930195729 fatty acid Natural products 0.000 description 4
- 210000001035 gastrointestinal tract Anatomy 0.000 description 4
- 239000003701 inert diluent Substances 0.000 description 4
- 229940047124 interferons Drugs 0.000 description 4
- 229910001629 magnesium chloride Inorganic materials 0.000 description 4
- 235000019359 magnesium stearate Nutrition 0.000 description 4
- 239000002207 metabolite Substances 0.000 description 4
- 239000000346 nonvolatile oil Substances 0.000 description 4
- LVTJOONKWUXEFR-FZRMHRINSA-N protoneodioscin Natural products O(C[C@@H](CC[C@]1(O)[C@H](C)[C@@H]2[C@]3(C)[C@H]([C@H]4[C@@H]([C@]5(C)C(=CC4)C[C@@H](O[C@@H]4[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@@H](O)[C@H](O[C@H]6[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O6)[C@H](CO)O4)CC5)CC3)C[C@@H]2O1)C)[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H](CO)O1 LVTJOONKWUXEFR-FZRMHRINSA-N 0.000 description 4
- 235000011152 sodium sulphate Nutrition 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- 239000005720 sucrose Substances 0.000 description 4
- 239000000829 suppository Substances 0.000 description 4
- 239000000375 suspending agent Substances 0.000 description 4
- 230000000699 topical effect Effects 0.000 description 4
- 239000000080 wetting agent Substances 0.000 description 4
- JENBYRMEJUIFGQ-UHFFFAOYSA-N 2-(6-chloropyridin-2-yl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)C1=CC=CC(Cl)=N1 JENBYRMEJUIFGQ-UHFFFAOYSA-N 0.000 description 3
- ZIDIKYIZXMYHAW-UHFFFAOYSA-N 2-bromo-6-fluoropyridine Chemical compound FC1=CC=CC(Br)=N1 ZIDIKYIZXMYHAW-UHFFFAOYSA-N 0.000 description 3
- YEDUAINPPJYDJZ-UHFFFAOYSA-N 2-hydroxybenzothiazole Chemical compound C1=CC=C2SC(O)=NC2=C1 YEDUAINPPJYDJZ-UHFFFAOYSA-N 0.000 description 3
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 3
- BABPUBPSCWEVAE-UHFFFAOYSA-N 4-(3,5-dimethyl-1h-pyrazol-4-yl)-1h-pyrrolo[2,3-b]pyridine Chemical compound CC1=NNC(C)=C1C1=CC=NC2=C1C=CN2 BABPUBPSCWEVAE-UHFFFAOYSA-N 0.000 description 3
- WUVVZVHSVCFVSW-UHFFFAOYSA-N 4-(furan-3-yl)-1h-pyrrolo[2,3-b]pyridine Chemical compound C1=CN=C2NC=CC2=C1C=1C=COC=1 WUVVZVHSVCFVSW-UHFFFAOYSA-N 0.000 description 3
- HJZFPRVFLBBAMU-UHFFFAOYSA-N 4-bromothiophene-2-carboxylic acid Chemical compound OC(=O)C1=CC(Br)=CS1 HJZFPRVFLBBAMU-UHFFFAOYSA-N 0.000 description 3
- IDQKIRACLMELKA-UHFFFAOYSA-N 4-pyrimidin-5-yl-1h-pyrrolo[2,3-b]pyridine Chemical compound C1=CN=C2NC=CC2=C1C1=CN=CN=C1 IDQKIRACLMELKA-UHFFFAOYSA-N 0.000 description 3
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- 239000005711 Benzoic acid Substances 0.000 description 3
- 229940126062 Compound A Drugs 0.000 description 3
- 229920002261 Corn starch Polymers 0.000 description 3
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 3
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 3
- 238000005481 NMR spectroscopy Methods 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 3
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 3
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 3
- 125000006615 aromatic heterocyclic group Chemical group 0.000 description 3
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 3
- 239000006172 buffering agent Substances 0.000 description 3
- 235000019437 butane-1,3-diol Nutrition 0.000 description 3
- 150000001721 carbon Chemical group 0.000 description 3
- 239000001768 carboxy methyl cellulose Substances 0.000 description 3
- 230000003197 catalytic effect Effects 0.000 description 3
- 210000004027 cell Anatomy 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 229940110456 cocoa butter Drugs 0.000 description 3
- 235000019868 cocoa butter Nutrition 0.000 description 3
- 229910052681 coesite Inorganic materials 0.000 description 3
- 239000008120 corn starch Substances 0.000 description 3
- 229940099112 cornstarch Drugs 0.000 description 3
- 229910052906 cristobalite Inorganic materials 0.000 description 3
- 229960004397 cyclophosphamide Drugs 0.000 description 3
- 230000003111 delayed effect Effects 0.000 description 3
- 230000002939 deleterious effect Effects 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 229960004979 fampridine Drugs 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 239000000796 flavoring agent Substances 0.000 description 3
- 238000004401 flow injection analysis Methods 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 150000002431 hydrogen Chemical class 0.000 description 3
- IOMMMLWIABWRKL-WUTDNEBXSA-N nazartinib Chemical compound C1N(C(=O)/C=C/CN(C)C)CCCC[C@H]1N1C2=C(Cl)C=CC=C2N=C1NC(=O)C1=CC=NC(C)=C1 IOMMMLWIABWRKL-WUTDNEBXSA-N 0.000 description 3
- 239000002674 ointment Substances 0.000 description 3
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 3
- 239000004006 olive oil Substances 0.000 description 3
- 235000019271 petrolatum Nutrition 0.000 description 3
- 235000021317 phosphate Nutrition 0.000 description 3
- 229910052698 phosphorus Inorganic materials 0.000 description 3
- 239000011574 phosphorus Substances 0.000 description 3
- 125000000587 piperidin-1-yl group Chemical group [H]C1([H])N(*)C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 3
- 235000011056 potassium acetate Nutrition 0.000 description 3
- 238000001556 precipitation Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 229960004063 propylene glycol Drugs 0.000 description 3
- FVAUCKIRQBBSSJ-UHFFFAOYSA-M sodium iodide Chemical compound [Na+].[I-] FVAUCKIRQBBSSJ-UHFFFAOYSA-M 0.000 description 3
- 239000008247 solid mixture Substances 0.000 description 3
- 229910052682 stishovite Inorganic materials 0.000 description 3
- 238000006467 substitution reaction Methods 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 239000003765 sweetening agent Substances 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- 229910052905 tridymite Inorganic materials 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- MFMMPFLTLDUQQZ-BYPYZUCNSA-N (2s)-2-[(2-chloro-5-fluoropyrimidin-4-yl)amino]-n-(2,2,2-trifluoroethyl)propanamide Chemical compound FC(F)(F)CNC(=O)[C@H](C)NC1=NC(Cl)=NC=C1F MFMMPFLTLDUQQZ-BYPYZUCNSA-N 0.000 description 2
- QAWXUPCNONFHQL-LURJTMIESA-N (2s)-2-[(6-bromopyridin-2-yl)amino]-n-(2,2,2-trifluoroethyl)propanamide Chemical compound FC(F)(F)CNC(=O)[C@H](C)NC1=CC=CC(Br)=N1 QAWXUPCNONFHQL-LURJTMIESA-N 0.000 description 2
- FNHHVPPSBFQMEL-KQHDFZBMSA-N (3S)-5-N-[(1S,5R)-3-hydroxy-6-bicyclo[3.1.0]hexanyl]-7-N,3-dimethyl-3-phenyl-2H-1-benzofuran-5,7-dicarboxamide Chemical compound CNC(=O)c1cc(cc2c1OC[C@@]2(C)c1ccccc1)C(=O)NC1[C@H]2CC(O)C[C@@H]12 FNHHVPPSBFQMEL-KQHDFZBMSA-N 0.000 description 2
- VXZHVNJQMHZXJB-UHFFFAOYSA-N (4-methylpiperazin-1-yl)-[3-(1h-pyrrolo[2,3-b]pyridin-4-yl)phenyl]methanone Chemical compound C1CN(C)CCN1C(=O)C1=CC=CC(C=2C=3C=CNC=3N=CC=2)=C1 VXZHVNJQMHZXJB-UHFFFAOYSA-N 0.000 description 2
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 description 2
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- KISWVXRQTGLFGD-UHFFFAOYSA-N 2-[[2-[[6-amino-2-[[2-[[2-[[5-amino-2-[[2-[[1-[2-[[6-amino-2-[(2,5-diamino-5-oxopentanoyl)amino]hexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]-5-(diaminomethylideneamino)p Chemical compound C1CCN(C(=O)C(CCCN=C(N)N)NC(=O)C(CCCCN)NC(=O)C(N)CCC(N)=O)C1C(=O)NC(CO)C(=O)NC(CCC(N)=O)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 KISWVXRQTGLFGD-UHFFFAOYSA-N 0.000 description 2
- HDECRAPHCDXMIJ-UHFFFAOYSA-N 2-methylbenzenesulfonyl chloride Chemical compound CC1=CC=CC=C1S(Cl)(=O)=O HDECRAPHCDXMIJ-UHFFFAOYSA-N 0.000 description 2
- BKOOMYPCSUNDGP-UHFFFAOYSA-N 2-methylbut-2-ene Chemical compound CC=C(C)C BKOOMYPCSUNDGP-UHFFFAOYSA-N 0.000 description 2
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 2
- KSNKQSPJFRQSEI-UHFFFAOYSA-N 3,3,3-trifluoropropanoic acid Chemical compound OC(=O)CC(F)(F)F KSNKQSPJFRQSEI-UHFFFAOYSA-N 0.000 description 2
- CPCMSNMGEMRMFF-UHFFFAOYSA-N 3-(3,3,3-trifluoropropylamino)propanenitrile Chemical compound FC(F)(F)CCNCCC#N CPCMSNMGEMRMFF-UHFFFAOYSA-N 0.000 description 2
- LULAYUGMBFYYEX-UHFFFAOYSA-N 3-chlorobenzoic acid Chemical compound OC(=O)C1=CC=CC(Cl)=C1 LULAYUGMBFYYEX-UHFFFAOYSA-N 0.000 description 2
- LZPWAYBEOJRFAX-UHFFFAOYSA-N 4,4,5,5-tetramethyl-1,3,2$l^{2}-dioxaborolane Chemical compound CC1(C)O[B]OC1(C)C LZPWAYBEOJRFAX-UHFFFAOYSA-N 0.000 description 2
- PDONIKHDXYHTLS-UHFFFAOYSA-N 4-bromothiophene-2-carbaldehyde Chemical compound BrC1=CSC(C=O)=C1 PDONIKHDXYHTLS-UHFFFAOYSA-N 0.000 description 2
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- 208000014997 Crohn colitis Diseases 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 108010005716 Interferon beta-1a Proteins 0.000 description 2
- UETNIIAIRMUTSM-UHFFFAOYSA-N Jacareubin Natural products CC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)O UETNIIAIRMUTSM-UHFFFAOYSA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 229910002651 NO3 Inorganic materials 0.000 description 2
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 2
- 239000005642 Oleic acid Substances 0.000 description 2
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 2
- 239000004264 Petrolatum Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N Propionic acid Chemical compound CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 150000003862 amino acid derivatives Chemical class 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 239000007900 aqueous suspension Substances 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 125000005160 aryl oxy alkyl group Chemical group 0.000 description 2
- DCBDOYDVQJVXOH-UHFFFAOYSA-N azane;1h-indole Chemical compound N.C1=CC=C2NC=CC2=C1 DCBDOYDVQJVXOH-UHFFFAOYSA-N 0.000 description 2
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 2
- 229960002170 azathioprine Drugs 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 235000019445 benzyl alcohol Nutrition 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- XMIIGOLPHOKFCH-UHFFFAOYSA-N beta-phenylpropanoic acid Natural products OC(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 238000004166 bioassay Methods 0.000 description 2
- 229920002988 biodegradable polymer Polymers 0.000 description 2
- 239000004621 biodegradable polymer Substances 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 229910000024 caesium carbonate Inorganic materials 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 239000004359 castor oil Substances 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 235000012343 cottonseed oil Nutrition 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 239000013058 crude material Substances 0.000 description 2
- SGJSQQLUMMGQOS-UHFFFAOYSA-N cyclohexanecarbonitrile Chemical compound N#C[C]1CCCCC1 SGJSQQLUMMGQOS-UHFFFAOYSA-N 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- SVPZJHKVRMRREG-UHFFFAOYSA-N cyclopentanecarbonitrile Chemical compound N#CC1CCCC1 SVPZJHKVRMRREG-UHFFFAOYSA-N 0.000 description 2
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 2
- AUQDITHEDVOTCU-UHFFFAOYSA-N cyclopropyl cyanide Chemical compound N#CC1CC1 AUQDITHEDVOTCU-UHFFFAOYSA-N 0.000 description 2
- 229940127089 cytotoxic agent Drugs 0.000 description 2
- UXGNZZKBCMGWAZ-UHFFFAOYSA-N dimethylformamide dmf Chemical compound CN(C)C=O.CN(C)C=O UXGNZZKBCMGWAZ-UHFFFAOYSA-N 0.000 description 2
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 2
- 229940043264 dodecyl sulfate Drugs 0.000 description 2
- ADEBPBSSDYVVLD-UHFFFAOYSA-N donepezil Chemical compound O=C1C=2C=C(OC)C(OC)=CC=2CC1CC(CC1)CCN1CC1=CC=CC=C1 ADEBPBSSDYVVLD-UHFFFAOYSA-N 0.000 description 2
- 239000008298 dragée Substances 0.000 description 2
- 239000003480 eluent Substances 0.000 description 2
- 238000010828 elution Methods 0.000 description 2
- 230000001804 emulsifying effect Effects 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 239000002702 enteric coating Substances 0.000 description 2
- 238000009505 enteric coating Methods 0.000 description 2
- MMXKVMNBHPAILY-UHFFFAOYSA-N ethyl laurate Chemical compound CCCCCCCCCCCC(=O)OCC MMXKVMNBHPAILY-UHFFFAOYSA-N 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 239000006260 foam Substances 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 238000007429 general method Methods 0.000 description 2
- FVIZARNDLVOMSU-UHFFFAOYSA-N ginsenoside K Natural products C1CC(C2(CCC3C(C)(C)C(O)CCC3(C)C2CC2O)C)(C)C2C1C(C)(CCC=C(C)C)OC1OC(CO)C(O)C(O)C1O FVIZARNDLVOMSU-UHFFFAOYSA-N 0.000 description 2
- 239000003365 glass fiber Substances 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 125000005456 glyceride group Chemical group 0.000 description 2
- 150000002334 glycols Chemical class 0.000 description 2
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 2
- 125000004475 heteroaralkyl group Chemical group 0.000 description 2
- 125000005114 heteroarylalkoxy group Chemical group 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical group 0.000 description 2
- 230000028993 immune response Effects 0.000 description 2
- 239000007972 injectable composition Substances 0.000 description 2
- 229940102223 injectable solution Drugs 0.000 description 2
- 229940102213 injectable suspension Drugs 0.000 description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 2
- 239000008297 liquid dosage form Substances 0.000 description 2
- 239000006210 lotion Substances 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- IZDROVVXIHRYMH-UHFFFAOYSA-N methanesulfonic anhydride Chemical compound CS(=O)(=O)OS(C)(=O)=O IZDROVVXIHRYMH-UHFFFAOYSA-N 0.000 description 2
- 239000004530 micro-emulsion Substances 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 125000000896 monocarboxylic acid group Chemical group 0.000 description 2
- DFOSXJAYPNNXPZ-UHFFFAOYSA-N n,n-dimethyl-1-[3-(1h-pyrrolo[2,3-b]pyridin-4-yl)phenyl]methanamine Chemical compound CN(C)CC1=CC=CC(C=2C=3C=CNC=3N=CC=2)=C1 DFOSXJAYPNNXPZ-UHFFFAOYSA-N 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- 231100000344 non-irritating Toxicity 0.000 description 2
- 235000008390 olive oil Nutrition 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 2
- 239000002304 perfume Substances 0.000 description 2
- 229940066842 petrolatum Drugs 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 125000004483 piperidin-3-yl group Chemical group N1CC(CCC1)* 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- IUBQJLUDMLPAGT-UHFFFAOYSA-N potassium bis(trimethylsilyl)amide Chemical compound C[Si](C)(C)N([K])[Si](C)(C)C IUBQJLUDMLPAGT-UHFFFAOYSA-N 0.000 description 2
- 210000002307 prostate Anatomy 0.000 description 2
- 125000006239 protecting group Chemical class 0.000 description 2
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 2
- 125000004528 pyrimidin-5-yl group Chemical group N1=CN=CC(=C1)* 0.000 description 2
- 238000005956 quaternization reaction Methods 0.000 description 2
- 210000000664 rectum Anatomy 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 239000012047 saturated solution Substances 0.000 description 2
- 239000008159 sesame oil Substances 0.000 description 2
- 235000011803 sesame oil Nutrition 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 210000003491 skin Anatomy 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 2
- NCEXYHBECQHGNR-QZQOTICOSA-N sulfasalazine Chemical compound C1=C(O)C(C(=O)O)=CC(\N=N\C=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-QZQOTICOSA-N 0.000 description 2
- 229960001940 sulfasalazine Drugs 0.000 description 2
- NCEXYHBECQHGNR-UHFFFAOYSA-N sulfasalazine Natural products C1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-UHFFFAOYSA-N 0.000 description 2
- YBBRCQOCSYXUOC-UHFFFAOYSA-N sulfuryl dichloride Chemical class ClS(Cl)(=O)=O YBBRCQOCSYXUOC-UHFFFAOYSA-N 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 235000012222 talc Nutrition 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 125000004001 thioalkyl group Chemical group 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- LSPHULWDVZXLIL-UHFFFAOYSA-N (+/-)-Camphoric acid Chemical compound CC1(C)C(C(O)=O)CCC1(C)C(O)=O LSPHULWDVZXLIL-UHFFFAOYSA-N 0.000 description 1
- ASGMFNBUXDJWJJ-JLCFBVMHSA-N (1R,3R)-3-[[3-bromo-1-[4-(5-methyl-1,3,4-thiadiazol-2-yl)phenyl]pyrazolo[3,4-d]pyrimidin-6-yl]amino]-N,1-dimethylcyclopentane-1-carboxamide Chemical compound BrC1=NN(C2=NC(=NC=C21)N[C@H]1C[C@@](CC1)(C(=O)NC)C)C1=CC=C(C=C1)C=1SC(=NN=1)C ASGMFNBUXDJWJJ-JLCFBVMHSA-N 0.000 description 1
- UAOUIVVJBYDFKD-XKCDOFEDSA-N (1R,9R,10S,11R,12R,15S,18S,21R)-10,11,21-trihydroxy-8,8-dimethyl-14-methylidene-4-(prop-2-enylamino)-20-oxa-5-thia-3-azahexacyclo[9.7.2.112,15.01,9.02,6.012,18]henicosa-2(6),3-dien-13-one Chemical compound C([C@@H]1[C@@H](O)[C@@]23C(C1=C)=O)C[C@H]2[C@]12C(N=C(NCC=C)S4)=C4CC(C)(C)[C@H]1[C@H](O)[C@]3(O)OC2 UAOUIVVJBYDFKD-XKCDOFEDSA-N 0.000 description 1
- ABJSOROVZZKJGI-OCYUSGCXSA-N (1r,2r,4r)-2-(4-bromophenyl)-n-[(4-chlorophenyl)-(2-fluoropyridin-4-yl)methyl]-4-morpholin-4-ylcyclohexane-1-carboxamide Chemical compound C1=NC(F)=CC(C(NC(=O)[C@H]2[C@@H](C[C@@H](CC2)N2CCOCC2)C=2C=CC(Br)=CC=2)C=2C=CC(Cl)=CC=2)=C1 ABJSOROVZZKJGI-OCYUSGCXSA-N 0.000 description 1
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 1
- IUSARDYWEPUTPN-OZBXUNDUSA-N (2r)-n-[(2s,3r)-4-[[(4s)-6-(2,2-dimethylpropyl)spiro[3,4-dihydropyrano[2,3-b]pyridine-2,1'-cyclobutane]-4-yl]amino]-3-hydroxy-1-[3-(1,3-thiazol-2-yl)phenyl]butan-2-yl]-2-methoxypropanamide Chemical compound C([C@H](NC(=O)[C@@H](C)OC)[C@H](O)CN[C@@H]1C2=CC(CC(C)(C)C)=CN=C2OC2(CCC2)C1)C(C=1)=CC=CC=1C1=NC=CS1 IUSARDYWEPUTPN-OZBXUNDUSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- YJLIKUSWRSEPSM-WGQQHEPDSA-N (2r,3r,4s,5r)-2-[6-amino-8-[(4-phenylphenyl)methylamino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C=1C=C(C=2C=CC=CC=2)C=CC=1CNC1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O YJLIKUSWRSEPSM-WGQQHEPDSA-N 0.000 description 1
- AJBOCBHMUQDLCQ-YFKPBYRVSA-N (2s)-2-[(2-chloropyrimidin-4-yl)amino]-n-(2,2,2-trifluoroethyl)propanamide Chemical compound FC(F)(F)CNC(=O)[C@H](C)NC1=CC=NC(Cl)=N1 AJBOCBHMUQDLCQ-YFKPBYRVSA-N 0.000 description 1
- QVHJQCGUWFKTSE-YFKPBYRVSA-N (2s)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid Chemical compound OC(=O)[C@H](C)NC(=O)OC(C)(C)C QVHJQCGUWFKTSE-YFKPBYRVSA-N 0.000 description 1
- VIJSPAIQWVPKQZ-BLECARSGSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-acetamido-5-(diaminomethylideneamino)pentanoyl]amino]-4-methylpentanoyl]amino]-4,4-dimethylpentanoyl]amino]-4-methylpentanoyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid Chemical compound NC(=N)NCCC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O VIJSPAIQWVPKQZ-BLECARSGSA-N 0.000 description 1
- STBLNCCBQMHSRC-BATDWUPUSA-N (2s)-n-[(3s,4s)-5-acetyl-7-cyano-4-methyl-1-[(2-methylnaphthalen-1-yl)methyl]-2-oxo-3,4-dihydro-1,5-benzodiazepin-3-yl]-2-(methylamino)propanamide Chemical compound O=C1[C@@H](NC(=O)[C@H](C)NC)[C@H](C)N(C(C)=O)C2=CC(C#N)=CC=C2N1CC1=C(C)C=CC2=CC=CC=C12 STBLNCCBQMHSRC-BATDWUPUSA-N 0.000 description 1
- RCVDPBFUMYUKPB-UHFFFAOYSA-N (3,4-dimethoxyphenyl)boronic acid Chemical compound COC1=CC=C(B(O)O)C=C1OC RCVDPBFUMYUKPB-UHFFFAOYSA-N 0.000 description 1
- IWZSHWBGHQBIML-ZGGLMWTQSA-N (3S,8S,10R,13S,14S,17S)-17-isoquinolin-7-yl-N,N,10,13-tetramethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-amine Chemical compound CN(C)[C@H]1CC[C@]2(C)C3CC[C@@]4(C)[C@@H](CC[C@@H]4c4ccc5ccncc5c4)[C@@H]3CC=C2C1 IWZSHWBGHQBIML-ZGGLMWTQSA-N 0.000 description 1
- UDQTXCHQKHIQMH-KYGLGHNPSA-N (3ar,5s,6s,7r,7ar)-5-(difluoromethyl)-2-(ethylamino)-5,6,7,7a-tetrahydro-3ah-pyrano[3,2-d][1,3]thiazole-6,7-diol Chemical compound S1C(NCC)=N[C@H]2[C@@H]1O[C@H](C(F)F)[C@@H](O)[C@@H]2O UDQTXCHQKHIQMH-KYGLGHNPSA-N 0.000 description 1
- HUWSZNZAROKDRZ-RRLWZMAJSA-N (3r,4r)-3-azaniumyl-5-[[(2s,3r)-1-[(2s)-2,3-dicarboxypyrrolidin-1-yl]-3-methyl-1-oxopentan-2-yl]amino]-5-oxo-4-sulfanylpentane-1-sulfonate Chemical compound OS(=O)(=O)CC[C@@H](N)[C@@H](S)C(=O)N[C@@H]([C@H](C)CC)C(=O)N1CCC(C(O)=O)[C@H]1C(O)=O HUWSZNZAROKDRZ-RRLWZMAJSA-N 0.000 description 1
- MPDDTAJMJCESGV-CTUHWIOQSA-M (3r,5r)-7-[2-(4-fluorophenyl)-5-[methyl-[(1r)-1-phenylethyl]carbamoyl]-4-propan-2-ylpyrazol-3-yl]-3,5-dihydroxyheptanoate Chemical compound C1([C@@H](C)N(C)C(=O)C2=NN(C(CC[C@@H](O)C[C@@H](O)CC([O-])=O)=C2C(C)C)C=2C=CC(F)=CC=2)=CC=CC=C1 MPDDTAJMJCESGV-CTUHWIOQSA-M 0.000 description 1
- YQOLEILXOBUDMU-KRWDZBQOSA-N (4R)-5-[(6-bromo-3-methyl-2-pyrrolidin-1-ylquinoline-4-carbonyl)amino]-4-(2-chlorophenyl)pentanoic acid Chemical compound CC1=C(C2=C(C=CC(=C2)Br)N=C1N3CCCC3)C(=O)NC[C@H](CCC(=O)O)C4=CC=CC=C4Cl YQOLEILXOBUDMU-KRWDZBQOSA-N 0.000 description 1
- STPKWKPURVSAJF-LJEWAXOPSA-N (4r,5r)-5-[4-[[4-(1-aza-4-azoniabicyclo[2.2.2]octan-4-ylmethyl)phenyl]methoxy]phenyl]-3,3-dibutyl-7-(dimethylamino)-1,1-dioxo-4,5-dihydro-2h-1$l^{6}-benzothiepin-4-ol Chemical compound O[C@H]1C(CCCC)(CCCC)CS(=O)(=O)C2=CC=C(N(C)C)C=C2[C@H]1C(C=C1)=CC=C1OCC(C=C1)=CC=C1C[N+]1(CC2)CCN2CC1 STPKWKPURVSAJF-LJEWAXOPSA-N 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- DEVSOMFAQLZNKR-RJRFIUFISA-N (z)-3-[3-[3,5-bis(trifluoromethyl)phenyl]-1,2,4-triazol-1-yl]-n'-pyrazin-2-ylprop-2-enehydrazide Chemical compound FC(F)(F)C1=CC(C(F)(F)F)=CC(C2=NN(\C=C/C(=O)NNC=3N=CC=NC=3)C=N2)=C1 DEVSOMFAQLZNKR-RJRFIUFISA-N 0.000 description 1
- 125000004502 1,2,3-oxadiazolyl group Chemical group 0.000 description 1
- 125000004511 1,2,3-thiadiazolyl group Chemical group 0.000 description 1
- 125000001399 1,2,3-triazolyl group Chemical group N1N=NC(=C1)* 0.000 description 1
- 125000004504 1,2,4-oxadiazolyl group Chemical group 0.000 description 1
- 125000004506 1,2,5-oxadiazolyl group Chemical group 0.000 description 1
- 125000004517 1,2,5-thiadiazolyl group Chemical group 0.000 description 1
- 125000004520 1,3,4-thiadiazolyl group Chemical group 0.000 description 1
- 125000003363 1,3,5-triazinyl group Chemical group N1=C(N=CN=C1)* 0.000 description 1
- 229940058015 1,3-butylene glycol Drugs 0.000 description 1
- PVOAHINGSUIXLS-UHFFFAOYSA-N 1-Methylpiperazine Chemical compound CN1CCNCC1 PVOAHINGSUIXLS-UHFFFAOYSA-N 0.000 description 1
- KQZLRWGGWXJPOS-NLFPWZOASA-N 1-[(1R)-1-(2,4-dichlorophenyl)ethyl]-6-[(4S,5R)-4-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]-5-methylcyclohexen-1-yl]pyrazolo[3,4-b]pyrazine-3-carbonitrile Chemical compound ClC1=C(C=CC(=C1)Cl)[C@@H](C)N1N=C(C=2C1=NC(=CN=2)C1=CC[C@@H]([C@@H](C1)C)N1[C@@H](CCC1)CO)C#N KQZLRWGGWXJPOS-NLFPWZOASA-N 0.000 description 1
- WZZBNLYBHUDSHF-DHLKQENFSA-N 1-[(3s,4s)-4-[8-(2-chloro-4-pyrimidin-2-yloxyphenyl)-7-fluoro-2-methylimidazo[4,5-c]quinolin-1-yl]-3-fluoropiperidin-1-yl]-2-hydroxyethanone Chemical compound CC1=NC2=CN=C3C=C(F)C(C=4C(=CC(OC=5N=CC=CN=5)=CC=4)Cl)=CC3=C2N1[C@H]1CCN(C(=O)CO)C[C@@H]1F WZZBNLYBHUDSHF-DHLKQENFSA-N 0.000 description 1
- QXOGPTXQGKQSJT-UHFFFAOYSA-N 1-amino-4-[4-(3,4-dimethylphenyl)sulfanylanilino]-9,10-dioxoanthracene-2-sulfonic acid Chemical compound Cc1ccc(Sc2ccc(Nc3cc(c(N)c4C(=O)c5ccccc5C(=O)c34)S(O)(=O)=O)cc2)cc1C QXOGPTXQGKQSJT-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- 125000001462 1-pyrrolyl group Chemical group [*]N1C([H])=C([H])C([H])=C1[H] 0.000 description 1
- DGHHQBMTXTWTJV-BQAIUKQQSA-N 119413-54-6 Chemical compound Cl.C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 DGHHQBMTXTWTJV-BQAIUKQQSA-N 0.000 description 1
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- YJUFGFXVASPYFQ-UHFFFAOYSA-N 2,3-dihydro-1-benzothiophene Chemical compound C1=CC=C2SCCC2=C1 YJUFGFXVASPYFQ-UHFFFAOYSA-N 0.000 description 1
- CHHHXKFHOYLYRE-UHFFFAOYSA-M 2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)- Chemical compound [K+].CC=CC=CC([O-])=O CHHHXKFHOYLYRE-UHFFFAOYSA-M 0.000 description 1
- BTTNYQZNBZNDOR-UHFFFAOYSA-N 2,4-dichloropyrimidine Chemical compound ClC1=CC=NC(Cl)=N1 BTTNYQZNBZNDOR-UHFFFAOYSA-N 0.000 description 1
- FILKGCRCWDMBKA-UHFFFAOYSA-N 2,6-dichloropyridine Chemical compound ClC1=CC=CC(Cl)=N1 FILKGCRCWDMBKA-UHFFFAOYSA-N 0.000 description 1
- YFTHTJAPODJVSL-UHFFFAOYSA-N 2-(1-benzothiophen-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Chemical compound O1C(C)(C)C(C)(C)OB1C1=CC=C(SC=C2)C2=C1 YFTHTJAPODJVSL-UHFFFAOYSA-N 0.000 description 1
- VCUXVXLUOHDHKK-UHFFFAOYSA-N 2-(2-aminopyrimidin-4-yl)-4-(2-chloro-4-methoxyphenyl)-1,3-thiazole-5-carboxamide Chemical compound ClC1=CC(OC)=CC=C1C1=C(C(N)=O)SC(C=2N=C(N)N=CC=2)=N1 VCUXVXLUOHDHKK-UHFFFAOYSA-N 0.000 description 1
- QEBYEVQKHRUYPE-UHFFFAOYSA-N 2-(2-chlorophenyl)-5-[(1-methylpyrazol-3-yl)methyl]-4-[[methyl(pyridin-3-ylmethyl)amino]methyl]-1h-pyrazolo[4,3-c]pyridine-3,6-dione Chemical compound C1=CN(C)N=C1CN1C(=O)C=C2NN(C=3C(=CC=CC=3)Cl)C(=O)C2=C1CN(C)CC1=CC=CN=C1 QEBYEVQKHRUYPE-UHFFFAOYSA-N 0.000 description 1
- WRMNZCZEMHIOCP-UHFFFAOYSA-N 2-Phenylethanol Natural products OCCC1=CC=CC=C1 WRMNZCZEMHIOCP-UHFFFAOYSA-N 0.000 description 1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 1
- VRHJBWUIWQOFLF-WLHGVMLRSA-N 2-[2-(4-benzo[b][1,4]benzothiazepin-6-ylpiperazin-1-yl)ethoxy]ethanol;(e)-but-2-enedioic acid Chemical compound OC(=O)\C=C\C(O)=O.C1CN(CCOCCO)CCN1C1=NC2=CC=CC=C2SC2=CC=CC=C12 VRHJBWUIWQOFLF-WLHGVMLRSA-N 0.000 description 1
- ADSCNOMKFNDGCD-UHFFFAOYSA-N 2-[3-(1h-pyrrolo[2,3-b]pyridin-4-yl)phenyl]acetonitrile Chemical compound N#CCC1=CC=CC(C=2C=3C=CNC=3N=CC=2)=C1 ADSCNOMKFNDGCD-UHFFFAOYSA-N 0.000 description 1
- UHTQHHLSGVOGQR-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-4-ium-1-yl]ethanesulfonate Chemical compound OCCN1CCN(CCS(O)(=O)=O)CC1.OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 UHTQHHLSGVOGQR-UHFFFAOYSA-N 0.000 description 1
- FMKGJQHNYMWDFJ-CVEARBPZSA-N 2-[[4-(2,2-difluoropropoxy)pyrimidin-5-yl]methylamino]-4-[[(1R,4S)-4-hydroxy-3,3-dimethylcyclohexyl]amino]pyrimidine-5-carbonitrile Chemical compound FC(COC1=NC=NC=C1CNC1=NC=C(C(=N1)N[C@H]1CC([C@H](CC1)O)(C)C)C#N)(C)F FMKGJQHNYMWDFJ-CVEARBPZSA-N 0.000 description 1
- YSUIQYOGTINQIN-UZFYAQMZSA-N 2-amino-9-[(1S,6R,8R,9S,10R,15R,17R,18R)-8-(6-aminopurin-9-yl)-9,18-difluoro-3,12-dihydroxy-3,12-bis(sulfanylidene)-2,4,7,11,13,16-hexaoxa-3lambda5,12lambda5-diphosphatricyclo[13.2.1.06,10]octadecan-17-yl]-1H-purin-6-one Chemical compound NC1=NC2=C(N=CN2[C@@H]2O[C@@H]3COP(S)(=O)O[C@@H]4[C@@H](COP(S)(=O)O[C@@H]2[C@@H]3F)O[C@H]([C@H]4F)N2C=NC3=C2N=CN=C3N)C(=O)N1 YSUIQYOGTINQIN-UZFYAQMZSA-N 0.000 description 1
- TVTJUIAKQFIXCE-HUKYDQBMSA-N 2-amino-9-[(2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-ynyl-1H-purine-6,8-dione Chemical compound NC=1NC(C=2N(C(N(C=2N=1)[C@@H]1O[C@@H]([C@H]([C@H]1O)F)CO)=O)CC#C)=O TVTJUIAKQFIXCE-HUKYDQBMSA-N 0.000 description 1
- JCBPETKZIGVZRE-UHFFFAOYSA-N 2-aminobutan-1-ol Chemical compound CCC(N)CO JCBPETKZIGVZRE-UHFFFAOYSA-N 0.000 description 1
- KQPQXZDIPWYWJO-UHFFFAOYSA-N 2-bromo-5-methoxythiophene Chemical compound COC1=CC=C(Br)S1 KQPQXZDIPWYWJO-UHFFFAOYSA-N 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- LFOIDLOIBZFWDO-UHFFFAOYSA-N 2-methoxy-6-[6-methoxy-4-[(3-phenylmethoxyphenyl)methoxy]-1-benzofuran-2-yl]imidazo[2,1-b][1,3,4]thiadiazole Chemical compound N1=C2SC(OC)=NN2C=C1C(OC1=CC(OC)=C2)=CC1=C2OCC(C=1)=CC=CC=1OCC1=CC=CC=C1 LFOIDLOIBZFWDO-UHFFFAOYSA-N 0.000 description 1
- 229940080296 2-naphthalenesulfonate Drugs 0.000 description 1
- LEACJMVNYZDSKR-UHFFFAOYSA-N 2-octyldodecan-1-ol Chemical compound CCCCCCCCCCC(CO)CCCCCCCC LEACJMVNYZDSKR-UHFFFAOYSA-N 0.000 description 1
- 125000004485 2-pyrrolidinyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])C1([H])* 0.000 description 1
- 125000000389 2-pyrrolyl group Chemical group [H]N1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- LGCYVLDNGBSOOW-UHFFFAOYSA-N 2H-benzotriazol-4-ol 1-hydroxybenzotriazole Chemical compound OC1=CC=CC2=C1N=NN2.C1=CC=C2N(O)N=NC2=C1 LGCYVLDNGBSOOW-UHFFFAOYSA-N 0.000 description 1
- FSUYMKXZLQOFQY-UHFFFAOYSA-N 3,4-dihydro-1,2-benzodithiine Chemical compound C1=CC=C2SSCCC2=C1 FSUYMKXZLQOFQY-UHFFFAOYSA-N 0.000 description 1
- DFRAKBCRUYUFNT-UHFFFAOYSA-N 3,8-dicyclohexyl-2,4,7,9-tetrahydro-[1,3]oxazino[5,6-h][1,3]benzoxazine Chemical compound C1CCCCC1N1CC(C=CC2=C3OCN(C2)C2CCCCC2)=C3OC1 DFRAKBCRUYUFNT-UHFFFAOYSA-N 0.000 description 1
- LFHFBDBZESNWLQ-UHFFFAOYSA-N 3-(1h-pyrrolo[2,3-b]pyridin-4-yl)phenol Chemical compound OC1=CC=CC(C=2C=3C=CNC=3N=CC=2)=C1 LFHFBDBZESNWLQ-UHFFFAOYSA-N 0.000 description 1
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 1
- WFOVEDJTASPCIR-UHFFFAOYSA-N 3-[(4-methyl-5-pyridin-4-yl-1,2,4-triazol-3-yl)methylamino]-n-[[2-(trifluoromethyl)phenyl]methyl]benzamide Chemical compound N=1N=C(C=2C=CN=CC=2)N(C)C=1CNC(C=1)=CC=CC=1C(=O)NCC1=CC=CC=C1C(F)(F)F WFOVEDJTASPCIR-UHFFFAOYSA-N 0.000 description 1
- BGAJNPLDJJBRHK-UHFFFAOYSA-N 3-[2-[5-(3-chloro-4-propan-2-yloxyphenyl)-1,3,4-thiadiazol-2-yl]-3-methyl-6,7-dihydro-4h-pyrazolo[4,3-c]pyridin-5-yl]propanoic acid Chemical compound C1=C(Cl)C(OC(C)C)=CC=C1C1=NN=C(N2C(=C3CN(CCC(O)=O)CCC3=N2)C)S1 BGAJNPLDJJBRHK-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- ZRPLANDPDWYOMZ-UHFFFAOYSA-N 3-cyclopentylpropionic acid Chemical compound OC(=O)CCC1CCCC1 ZRPLANDPDWYOMZ-UHFFFAOYSA-N 0.000 description 1
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- XMIIGOLPHOKFCH-UHFFFAOYSA-M 3-phenylpropionate Chemical compound [O-]C(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-M 0.000 description 1
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000004575 3-pyrrolidinyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001397 3-pyrrolyl group Chemical group [H]N1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- CSVKEFBFKJBWND-UHFFFAOYSA-N 4,4,4-trifluoro-2-methylbutanamide Chemical compound NC(=O)C(C)CC(F)(F)F CSVKEFBFKJBWND-UHFFFAOYSA-N 0.000 description 1
- JAOINXWEFKZYIL-UHFFFAOYSA-N 4-(1-methyl-2-oxoquinolin-4-yl)oxy-n-(4-methylpyridin-2-yl)butanamide;2,2,2-trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.CC1=CC=NC(NC(=O)CCCOC=2C3=CC=CC=C3N(C)C(=O)C=2)=C1 JAOINXWEFKZYIL-UHFFFAOYSA-N 0.000 description 1
- CAKKWSHZBOSGPV-UHFFFAOYSA-N 4-(3,5-dimethylphenyl)-1h-pyrrolo[2,3-b]pyridine Chemical compound CC1=CC(C)=CC(C=2C=3C=CNC=3N=CC=2)=C1 CAKKWSHZBOSGPV-UHFFFAOYSA-N 0.000 description 1
- JSNCRUDROUTYBO-UHFFFAOYSA-N 4-(6-fluoropyridin-2-yl)-1h-pyrrolo[2,3-b]pyridine Chemical compound FC1=CC=CC(C=2C=3C=CNC=3N=CC=2)=N1 JSNCRUDROUTYBO-UHFFFAOYSA-N 0.000 description 1
- XWRZDBIWCPESJH-UHFFFAOYSA-N 4-[3-(trifluoromethyl)phenyl]-1h-pyrrolo[2,3-b]pyridine Chemical compound FC(F)(F)C1=CC=CC(C=2C=3C=CNC=3N=CC=2)=C1 XWRZDBIWCPESJH-UHFFFAOYSA-N 0.000 description 1
- WYFCZWSWFGJODV-MIANJLSGSA-N 4-[[(1s)-2-[(e)-3-[3-chloro-2-fluoro-6-(tetrazol-1-yl)phenyl]prop-2-enoyl]-5-(4-methyl-2-oxopiperazin-1-yl)-3,4-dihydro-1h-isoquinoline-1-carbonyl]amino]benzoic acid Chemical compound O=C1CN(C)CCN1C1=CC=CC2=C1CCN(C(=O)\C=C\C=1C(=CC=C(Cl)C=1F)N1N=NN=C1)[C@@H]2C(=O)NC1=CC=C(C(O)=O)C=C1 WYFCZWSWFGJODV-MIANJLSGSA-N 0.000 description 1
- 125000004203 4-hydroxyphenyl group Chemical group [H]OC1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- VTDLIXTUIXEFID-UHFFFAOYSA-N 4-pyridin-3-yl-1h-pyrrolo[2,3-b]pyridine Chemical compound C1=CN=C2NC=CC2=C1C1=CC=CN=C1 VTDLIXTUIXEFID-UHFFFAOYSA-N 0.000 description 1
- KDDQRKBRJSGMQE-UHFFFAOYSA-N 4-thiazolyl Chemical group [C]1=CSC=N1 KDDQRKBRJSGMQE-UHFFFAOYSA-N 0.000 description 1
- VKLKXFOZNHEBSW-UHFFFAOYSA-N 5-[[3-[(4-morpholin-4-ylbenzoyl)amino]phenyl]methoxy]pyridine-3-carboxamide Chemical compound O1CCN(CC1)C1=CC=C(C(=O)NC=2C=C(COC=3C=NC=C(C(=O)N)C=3)C=CC=2)C=C1 VKLKXFOZNHEBSW-UHFFFAOYSA-N 0.000 description 1
- XFJBGINZIMNZBW-CRAIPNDOSA-N 5-chloro-2-[4-[(1r,2s)-2-[2-(5-methylsulfonylpyridin-2-yl)oxyethyl]cyclopropyl]piperidin-1-yl]pyrimidine Chemical compound N1=CC(S(=O)(=O)C)=CC=C1OCC[C@H]1[C@@H](C2CCN(CC2)C=2N=CC(Cl)=CN=2)C1 XFJBGINZIMNZBW-CRAIPNDOSA-N 0.000 description 1
- 125000006163 5-membered heteroaryl group Chemical group 0.000 description 1
- CWDWFSXUQODZGW-UHFFFAOYSA-N 5-thiazolyl Chemical group [C]1=CN=CS1 CWDWFSXUQODZGW-UHFFFAOYSA-N 0.000 description 1
- QVJDIGLZFCZAKK-UHFFFAOYSA-N 6-[1-(4-methylphenyl)sulfonylpyrrolo[2,3-b]pyridin-4-yl]pyridin-2-amine Chemical compound C1=CC(C)=CC=C1S(=O)(=O)N1C2=NC=CC(C=3N=C(N)C=CC=3)=C2C=C1 QVJDIGLZFCZAKK-UHFFFAOYSA-N 0.000 description 1
- GDUANFXPOZTYKS-UHFFFAOYSA-N 6-bromo-8-[(2,6-difluoro-4-methoxybenzoyl)amino]-4-oxochromene-2-carboxylic acid Chemical compound FC1=CC(OC)=CC(F)=C1C(=O)NC1=CC(Br)=CC2=C1OC(C(O)=O)=CC2=O GDUANFXPOZTYKS-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- HCCNBKFJYUWLEX-UHFFFAOYSA-N 7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(pyrazin-2-ylmethylamino)pyrido[3,4-b]pyrazin-2-one Chemical compound O=C1N(CCOCCC)C2=CC(C=3C=NC(OC)=CC=3)=NC=C2N=C1NCC1=CN=CC=N1 HCCNBKFJYUWLEX-UHFFFAOYSA-N 0.000 description 1
- XASOHFCUIQARJT-UHFFFAOYSA-N 8-methoxy-6-[7-(2-morpholin-4-ylethoxy)imidazo[1,2-a]pyridin-3-yl]-2-(2,2,2-trifluoroethyl)-3,4-dihydroisoquinolin-1-one Chemical compound C(N1C(=O)C2=C(OC)C=C(C=3N4C(=NC=3)C=C(C=C4)OCCN3CCOCC3)C=C2CC1)C(F)(F)F XASOHFCUIQARJT-UHFFFAOYSA-N 0.000 description 1
- IRBAWVGZNJIROV-SFHVURJKSA-N 9-(2-cyclopropylethynyl)-2-[[(2s)-1,4-dioxan-2-yl]methoxy]-6,7-dihydropyrimido[6,1-a]isoquinolin-4-one Chemical compound C1=C2C3=CC=C(C#CC4CC4)C=C3CCN2C(=O)N=C1OC[C@@H]1COCCO1 IRBAWVGZNJIROV-SFHVURJKSA-N 0.000 description 1
- 239000005541 ACE inhibitor Substances 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- 235000003276 Apios tuberosa Nutrition 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000010744 Arachis villosulicarpa Nutrition 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 208000037398 BCR-ABL1 negative atypical chronic myeloid leukemia Diseases 0.000 description 1
- IYHHRZBKXXKDDY-UHFFFAOYSA-N BI-605906 Chemical compound N=1C=2SC(C(N)=O)=C(N)C=2C(C(F)(F)CC)=CC=1N1CCC(S(C)(=O)=O)CC1 IYHHRZBKXXKDDY-UHFFFAOYSA-N 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 238000007125 Buchwald synthesis reaction Methods 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 1
- QNGTVGAIEOIQBR-UHFFFAOYSA-N CC(C)(C)OC(Nc1cc(Br)c[s]1)=O Chemical compound CC(C)(C)OC(Nc1cc(Br)c[s]1)=O QNGTVGAIEOIQBR-UHFFFAOYSA-N 0.000 description 1
- KCBAMQOKOLXLOX-BSZYMOERSA-N CC1=C(SC=N1)C2=CC=C(C=C2)[C@H](C)NC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNCCCONC(=O)C4=C(C(=C(C=C4)F)F)NC5=C(C=C(C=C5)I)F)O Chemical compound CC1=C(SC=N1)C2=CC=C(C=C2)[C@H](C)NC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNCCCONC(=O)C4=C(C(=C(C=C4)F)F)NC5=C(C=C(C=C5)I)F)O KCBAMQOKOLXLOX-BSZYMOERSA-N 0.000 description 1
- UHNRLQRZRNKOKU-UHFFFAOYSA-N CCN(CC1=NC2=C(N1)C1=CC=C(C=C1N=C2N)C1=NNC=C1)C(C)=O Chemical compound CCN(CC1=NC2=C(N1)C1=CC=C(C=C1N=C2N)C1=NNC=C1)C(C)=O UHNRLQRZRNKOKU-UHFFFAOYSA-N 0.000 description 1
- BQXUPNKLZNSUMC-YUQWMIPFSA-N CCN(CCCCCOCC(=O)N[C@H](C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](C)c1ccc(cc1)-c1scnc1C)C(C)(C)C)CCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Chemical compound CCN(CCCCCOCC(=O)N[C@H](C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](C)c1ccc(cc1)-c1scnc1C)C(C)(C)C)CCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 BQXUPNKLZNSUMC-YUQWMIPFSA-N 0.000 description 1
- 101150041968 CDC13 gene Proteins 0.000 description 1
- DRSHXJFUUPIBHX-UHFFFAOYSA-N COc1ccc(cc1)N1N=CC2C=NC(Nc3cc(OC)c(OC)c(OCCCN4CCN(C)CC4)c3)=NC12 Chemical compound COc1ccc(cc1)N1N=CC2C=NC(Nc3cc(OC)c(OC)c(OCCCN4CCN(C)CC4)c3)=NC12 DRSHXJFUUPIBHX-UHFFFAOYSA-N 0.000 description 1
- NCBZRJODKRCREW-UHFFFAOYSA-N COc1cccc(N)c1 Chemical compound COc1cccc(N)c1 NCBZRJODKRCREW-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 229940127291 Calcium channel antagonist Drugs 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 229920001268 Cholestyramine Polymers 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 240000004307 Citrus medica Species 0.000 description 1
- 229940127007 Compound 39 Drugs 0.000 description 1
- HTJDQJBWANPRPF-UHFFFAOYSA-N Cyclopropylamine Chemical compound NC1CC1 HTJDQJBWANPRPF-UHFFFAOYSA-N 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 229930105110 Cyclosporin A Natural products 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 102000054300 EC 2.7.11.- Human genes 0.000 description 1
- 108700035490 EC 2.7.11.- Proteins 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- GNIMZPARQHMPGN-UHFFFAOYSA-N Fc1cc(N(Cc2ccccc2)Cc2ccccc2)cc(F)c1I Chemical compound Fc1cc(N(Cc2ccccc2)Cc2ccccc2)cc(F)c1I GNIMZPARQHMPGN-UHFFFAOYSA-N 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 241000206672 Gelidium Species 0.000 description 1
- 108010072051 Glatiramer Acetate Proteins 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Polymers OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000844245 Homo sapiens Non-receptor tyrosine-protein kinase TYK2 Proteins 0.000 description 1
- 101001059454 Homo sapiens Serine/threonine-protein kinase MARK2 Proteins 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 102000003996 Interferon-beta Human genes 0.000 description 1
- 108090000862 Ion Channels Proteins 0.000 description 1
- 102000004310 Ion Channels Human genes 0.000 description 1
- 229940123241 Janus kinase 3 inhibitor Drugs 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- WRHZVMBBRYBTKZ-UHFFFAOYSA-N Minaline Natural products OC(=O)C1=CC=CN1 WRHZVMBBRYBTKZ-UHFFFAOYSA-N 0.000 description 1
- UCHDWCPVSPXUMX-TZIWLTJVSA-N Montelukast Chemical compound CC(C)(O)C1=CC=CC=C1CC[C@H](C=1C=C(\C=C\C=2N=C3C=C(Cl)C=CC3=CC=2)C=CC=1)SCC1(CC(O)=O)CC1 UCHDWCPVSPXUMX-TZIWLTJVSA-N 0.000 description 1
- 102000047918 Myelin Basic Human genes 0.000 description 1
- 101710107068 Myelin basic protein Proteins 0.000 description 1
- 208000037538 Myelomonocytic Juvenile Leukemia Diseases 0.000 description 1
- LVDRREOUMKACNJ-BKMJKUGQSA-N N-[(2R,3S)-2-(4-chlorophenyl)-1-(1,4-dimethyl-2-oxoquinolin-7-yl)-6-oxopiperidin-3-yl]-2-methylpropane-1-sulfonamide Chemical compound CC(C)CS(=O)(=O)N[C@H]1CCC(=O)N([C@@H]1c1ccc(Cl)cc1)c1ccc2c(C)cc(=O)n(C)c2c1 LVDRREOUMKACNJ-BKMJKUGQSA-N 0.000 description 1
- GXCLVBGFBYZDAG-UHFFFAOYSA-N N-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amine Chemical compound CN(CCC1=CNC2=C1C=CC=C2)CC=C GXCLVBGFBYZDAG-UHFFFAOYSA-N 0.000 description 1
- AVYVHIKSFXVDBG-UHFFFAOYSA-N N-benzyl-N-hydroxy-2,2-dimethylbutanamide Chemical compound C(C1=CC=CC=C1)N(C(C(CC)(C)C)=O)O AVYVHIKSFXVDBG-UHFFFAOYSA-N 0.000 description 1
- POFVJRKJJBFPII-UHFFFAOYSA-N N-cyclopentyl-5-[2-[[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]amino]-5-fluoropyrimidin-4-yl]-4-methyl-1,3-thiazol-2-amine Chemical compound C1(CCCC1)NC=1SC(=C(N=1)C)C1=NC(=NC=C1F)NC1=NC=C(C=C1)CN1CCN(CC1)CC POFVJRKJJBFPII-UHFFFAOYSA-N 0.000 description 1
- OPFJDXRVMFKJJO-ZHHKINOHSA-N N-{[3-(2-benzamido-4-methyl-1,3-thiazol-5-yl)-pyrazol-5-yl]carbonyl}-G-dR-G-dD-dD-dD-NH2 Chemical compound S1C(C=2NN=C(C=2)C(=O)NCC(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(N)=O)=C(C)N=C1NC(=O)C1=CC=CC=C1 OPFJDXRVMFKJJO-ZHHKINOHSA-N 0.000 description 1
- CUYKNJBYIJFRCU-UHFFFAOYSA-N Nc1cnccc1 Chemical compound Nc1cnccc1 CUYKNJBYIJFRCU-UHFFFAOYSA-N 0.000 description 1
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 1
- 102100032028 Non-receptor tyrosine-protein kinase TYK2 Human genes 0.000 description 1
- 240000007817 Olea europaea Species 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 229910002666 PdCl2 Inorganic materials 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102000007327 Protamines Human genes 0.000 description 1
- 108010007568 Protamines Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 101000729528 Rattus norvegicus Rho-associated protein kinase 2 Proteins 0.000 description 1
- 102100039314 Rho-associated protein kinase 2 Human genes 0.000 description 1
- 101710088493 Rho-associated protein kinase 2 Proteins 0.000 description 1
- 235000004443 Ricinus communis Nutrition 0.000 description 1
- FTALBRSUTCGOEG-UHFFFAOYSA-N Riluzole Chemical compound C1=C(OC(F)(F)F)C=C2SC(N)=NC2=C1 FTALBRSUTCGOEG-UHFFFAOYSA-N 0.000 description 1
- 235000019485 Safflower oil Nutrition 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 1
- 102000018410 Small GTPase Rho Human genes 0.000 description 1
- 108050007506 Small GTPase Rho Proteins 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 1
- 208000005392 Spasm Diseases 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 239000012317 TBTU Substances 0.000 description 1
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 101710112791 Tyrosine-protein kinase JAK2 Proteins 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- LJOOWESTVASNOG-UFJKPHDISA-N [(1s,3r,4ar,7s,8s,8as)-3-hydroxy-8-[2-[(4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-yl] (2s)-2-methylbutanoate Chemical compound C([C@H]1[C@@H](C)C=C[C@H]2C[C@@H](O)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)CC1C[C@@H](O)CC(=O)O1 LJOOWESTVASNOG-UFJKPHDISA-N 0.000 description 1
- SPXSEZMVRJLHQG-XMMPIXPASA-N [(2R)-1-[[4-[(3-phenylmethoxyphenoxy)methyl]phenyl]methyl]pyrrolidin-2-yl]methanol Chemical compound C(C1=CC=CC=C1)OC=1C=C(OCC2=CC=C(CN3[C@H](CCC3)CO)C=C2)C=CC=1 SPXSEZMVRJLHQG-XMMPIXPASA-N 0.000 description 1
- IOSLINNLJFQMFF-XMMPIXPASA-N [(2R)-1-[[4-[[3-[(4-fluorophenyl)methylsulfanyl]phenoxy]methyl]phenyl]methyl]pyrrolidin-2-yl]methanol Chemical compound FC1=CC=C(CSC=2C=C(OCC3=CC=C(CN4[C@H](CCC4)CO)C=C3)C=CC=2)C=C1 IOSLINNLJFQMFF-XMMPIXPASA-N 0.000 description 1
- 239000001089 [(2R)-oxolan-2-yl]methanol Substances 0.000 description 1
- MXZNUGFCDVAXLG-CHWSQXEVSA-N [(2S)-1-[(2R)-3-methyl-2-(pyridine-4-carbonylamino)butanoyl]pyrrolidin-2-yl]boronic acid Chemical compound CC(C)[C@@H](NC(=O)c1ccncc1)C(=O)N1CCC[C@@H]1B(O)O MXZNUGFCDVAXLG-CHWSQXEVSA-N 0.000 description 1
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 1
- SZPWXAOBLNYOHY-UHFFFAOYSA-N [C]1=CC=NC2=CC=CC=C12 Chemical group [C]1=CC=NC2=CC=CC=C12 SZPWXAOBLNYOHY-UHFFFAOYSA-N 0.000 description 1
- SMNRFWMNPDABKZ-WVALLCKVSA-N [[(2R,3S,4R,5S)-5-(2,6-dioxo-3H-pyridin-3-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [[[(2R,3S,4S,5R,6R)-4-fluoro-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl] hydrogen phosphate Chemical compound OC[C@H]1O[C@H](OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)C2C=CC(=O)NC2=O)[C@H](O)[C@@H](F)[C@@H]1O SMNRFWMNPDABKZ-WVALLCKVSA-N 0.000 description 1
- CTCBPRXHVPZNHB-VQFZJOCSSA-N [[(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate;(2r,3r,4s,5r)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O.C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O CTCBPRXHVPZNHB-VQFZJOCSSA-N 0.000 description 1
- SORGEQQSQGNZFI-UHFFFAOYSA-N [azido(phenoxy)phosphoryl]oxybenzene Chemical compound C=1C=CC=CC=1OP(=O)(N=[N+]=[N-])OC1=CC=CC=C1 SORGEQQSQGNZFI-UHFFFAOYSA-N 0.000 description 1
- CLZISMQKJZCZDN-UHFFFAOYSA-N [benzotriazol-1-yloxy(dimethylamino)methylidene]-dimethylazanium Chemical compound C1=CC=C2N(OC(N(C)C)=[N+](C)C)N=NC2=C1 CLZISMQKJZCZDN-UHFFFAOYSA-N 0.000 description 1
- FYJKEHKQUPSJDH-UHFFFAOYSA-N [dimethyl-(trimethylsilylamino)silyl]methane;potassium Chemical compound [K].C[Si](C)(C)N[Si](C)(C)C FYJKEHKQUPSJDH-UHFFFAOYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 229940124532 absorption promoter Drugs 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- PQLVXDKIJBQVDF-UHFFFAOYSA-N acetic acid;hydrate Chemical compound O.CC(O)=O PQLVXDKIJBQVDF-UHFFFAOYSA-N 0.000 description 1
- PBCJIPOGFJYBJE-UHFFFAOYSA-N acetonitrile;hydrate Chemical compound O.CC#N PBCJIPOGFJYBJE-UHFFFAOYSA-N 0.000 description 1
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 1
- 239000012346 acetyl chloride Substances 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-L adipate(2-) Chemical compound [O-]C(=O)CCCCC([O-])=O WNLRTRBMVRJNCN-UHFFFAOYSA-L 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- CEGOLXSVJUTHNZ-UHFFFAOYSA-K aluminium tristearate Chemical compound [Al+3].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CEGOLXSVJUTHNZ-UHFFFAOYSA-K 0.000 description 1
- 229940063655 aluminum stearate Drugs 0.000 description 1
- DKNWSYNQZKUICI-UHFFFAOYSA-N amantadine Chemical compound C1C(C2)CC3CC2CC1(N)C3 DKNWSYNQZKUICI-UHFFFAOYSA-N 0.000 description 1
- 229960003805 amantadine Drugs 0.000 description 1
- 229940024606 amino acid Drugs 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 description 1
- 230000001773 anti-convulsant effect Effects 0.000 description 1
- 229940125681 anticonvulsant agent Drugs 0.000 description 1
- 239000001961 anticonvulsive agent Substances 0.000 description 1
- 229940082992 antihypertensives mao inhibitors Drugs 0.000 description 1
- 239000000063 antileukemic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 239000000939 antiparkinson agent Substances 0.000 description 1
- 229940039856 aricept Drugs 0.000 description 1
- 150000004982 aromatic amines Chemical class 0.000 description 1
- 125000005228 aryl sulfonate group Chemical group 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- XRWSZZJLZRKHHD-WVWIJVSJSA-N asunaprevir Chemical compound O=C([C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)OC1=NC=C(C2=CC=C(Cl)C=C21)OC)N[C@]1(C(=O)NS(=O)(=O)C2CC2)C[C@H]1C=C XRWSZZJLZRKHHD-WVWIJVSJSA-N 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 201000004892 atypical chronic myeloid leukemia Diseases 0.000 description 1
- 229940003504 avonex Drugs 0.000 description 1
- MVXVYAKCVDQRLW-UHFFFAOYSA-N azain Natural products C1=CN=C2NC=CC2=C1 MVXVYAKCVDQRLW-UHFFFAOYSA-N 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- KXDAEFPNCMNJSK-UHFFFAOYSA-N benzene carboxamide Natural products NC(=O)C1=CC=CC=C1 KXDAEFPNCMNJSK-UHFFFAOYSA-N 0.000 description 1
- 229940077388 benzenesulfonate Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 229940050390 benzoate Drugs 0.000 description 1
- 125000004618 benzofuryl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- KCXMKQUNVWSEMD-UHFFFAOYSA-N benzyl chloride Chemical compound ClCC1=CC=CC=C1 KCXMKQUNVWSEMD-UHFFFAOYSA-N 0.000 description 1
- 229940073608 benzyl chloride Drugs 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- OZVBMTJYIDMWIL-AYFBDAFISA-N bromocriptine Chemical compound C1=CC(C=2[C@H](N(C)C[C@@H](C=2)C(=O)N[C@]2(C(=O)N3[C@H](C(N4CCC[C@H]4[C@]3(O)O2)=O)CC(C)C)C(C)C)C2)=C3C2=C(Br)NC3=C1 OZVBMTJYIDMWIL-AYFBDAFISA-N 0.000 description 1
- 229960002802 bromocriptine Drugs 0.000 description 1
- DQXBYHZEEUGOBF-UHFFFAOYSA-N but-3-enoic acid;ethene Chemical compound C=C.OC(=O)CC=C DQXBYHZEEUGOBF-UHFFFAOYSA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000000480 calcium channel blocker Substances 0.000 description 1
- FATUQANACHZLRT-KMRXSBRUSA-L calcium glucoheptonate Chemical compound [Ca+2].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)C([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)C([O-])=O FATUQANACHZLRT-KMRXSBRUSA-L 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical compound C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 229960004205 carbidopa Drugs 0.000 description 1
- TZFNLOMSOLWIDK-JTQLQIEISA-N carbidopa (anhydrous) Chemical compound NN[C@@](C(O)=O)(C)CC1=CC=C(O)C(O)=C1 TZFNLOMSOLWIDK-JTQLQIEISA-N 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 239000012876 carrier material Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 229940081733 cetearyl alcohol Drugs 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 229910052729 chemical element Inorganic materials 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- FZFAMSAMCHXGEF-UHFFFAOYSA-N chloro formate Chemical compound ClOC=O FZFAMSAMCHXGEF-UHFFFAOYSA-N 0.000 description 1
- AOGYCOYQMAVAFD-UHFFFAOYSA-N chlorocarbonic acid Chemical class OC(Cl)=O AOGYCOYQMAVAFD-UHFFFAOYSA-N 0.000 description 1
- 239000000544 cholinesterase inhibitor Substances 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- 229960001653 citalopram Drugs 0.000 description 1
- 108010060348 citron-kinase Proteins 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 230000035602 clotting Effects 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 238000010835 comparative analysis Methods 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 229940126543 compound 14 Drugs 0.000 description 1
- 229940125810 compound 20 Drugs 0.000 description 1
- 229940126086 compound 21 Drugs 0.000 description 1
- 229940126208 compound 22 Drugs 0.000 description 1
- 229940125833 compound 23 Drugs 0.000 description 1
- 229940125961 compound 24 Drugs 0.000 description 1
- 229940125851 compound 27 Drugs 0.000 description 1
- 229940127204 compound 29 Drugs 0.000 description 1
- 229940126214 compound 3 Drugs 0.000 description 1
- 229940125877 compound 31 Drugs 0.000 description 1
- 229940125878 compound 36 Drugs 0.000 description 1
- 229940125807 compound 37 Drugs 0.000 description 1
- 229940127573 compound 38 Drugs 0.000 description 1
- 229940126540 compound 41 Drugs 0.000 description 1
- 229940125936 compound 42 Drugs 0.000 description 1
- 229940125844 compound 46 Drugs 0.000 description 1
- 229940127271 compound 49 Drugs 0.000 description 1
- 229940126545 compound 53 Drugs 0.000 description 1
- 229940127113 compound 57 Drugs 0.000 description 1
- 229940125900 compound 59 Drugs 0.000 description 1
- ONCCWDRMOZMNSM-FBCQKBJTSA-N compound Z Chemical compound N1=C2C(=O)NC(N)=NC2=NC=C1C(=O)[C@H]1OP(O)(=O)OC[C@H]1O ONCCWDRMOZMNSM-FBCQKBJTSA-N 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 239000012050 conventional carrier Substances 0.000 description 1
- 229940038717 copaxone Drugs 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 239000012045 crude solution Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 125000000392 cycloalkenyl group Chemical group 0.000 description 1
- 125000001162 cycloheptenyl group Chemical group C1(=CCCCCC1)* 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 1
- NISGSNTVMOOSJQ-UHFFFAOYSA-N cyclopentanamine Chemical compound NC1CCCC1 NISGSNTVMOOSJQ-UHFFFAOYSA-N 0.000 description 1
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- DEZRYPDIMOWBDS-UHFFFAOYSA-N dcm dichloromethane Chemical compound ClCCl.ClCCl DEZRYPDIMOWBDS-UHFFFAOYSA-N 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- YNHIGQDRGKUECZ-UHFFFAOYSA-N dichloropalladium;triphenylphosphanium Chemical compound Cl[Pd]Cl.C1=CC=CC=C1[PH+](C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1[PH+](C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-N 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- GXGAKHNRMVGRPK-UHFFFAOYSA-N dimagnesium;dioxido-bis[[oxido(oxo)silyl]oxy]silane Chemical compound [Mg+2].[Mg+2].[O-][Si](=O)O[Si]([O-])([O-])O[Si]([O-])=O GXGAKHNRMVGRPK-UHFFFAOYSA-N 0.000 description 1
- 125000004311 dioxin-2-yl group Chemical group [H]C1=C([H])OC(*)=C([H])O1 0.000 description 1
- MKRTXPORKIRPDG-UHFFFAOYSA-N diphenylphosphoryl azide Chemical compound C=1C=CC=CC=1P(=O)(N=[N+]=[N-])C1=CC=CC=C1 MKRTXPORKIRPDG-UHFFFAOYSA-N 0.000 description 1
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 1
- 229910000396 dipotassium phosphate Inorganic materials 0.000 description 1
- 235000019797 dipotassium phosphate Nutrition 0.000 description 1
- 208000037765 diseases and disorders Diseases 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- UZZWBUYVTBPQIV-UHFFFAOYSA-N dme dimethoxyethane Chemical compound COCCOC.COCCOC UZZWBUYVTBPQIV-UHFFFAOYSA-N 0.000 description 1
- CETRZFQIITUQQL-UHFFFAOYSA-N dmso dimethylsulfoxide Chemical compound CS(C)=O.CS(C)=O CETRZFQIITUQQL-UHFFFAOYSA-N 0.000 description 1
- POULHZVOKOAJMA-UHFFFAOYSA-M dodecanoate Chemical compound CCCCCCCCCCCC([O-])=O POULHZVOKOAJMA-UHFFFAOYSA-M 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000003221 ear drop Substances 0.000 description 1
- 229940047652 ear drops Drugs 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 239000008387 emulsifying waxe Substances 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940095399 enema Drugs 0.000 description 1
- JRURYQJSLYLRLN-BJMVGYQFSA-N entacapone Chemical compound CCN(CC)C(=O)C(\C#N)=C\C1=CC(O)=C(O)C([N+]([O-])=O)=C1 JRURYQJSLYLRLN-BJMVGYQFSA-N 0.000 description 1
- 229960003337 entacapone Drugs 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- FPEUIONIYRKUDE-UHFFFAOYSA-N ethyl 2-(6-chloropyridin-2-yl)-2-methylpropanoate Chemical compound CCOC(=O)C(C)(C)C1=CC=CC(Cl)=N1 FPEUIONIYRKUDE-UHFFFAOYSA-N 0.000 description 1
- GWNFQAKCJYEJEW-UHFFFAOYSA-N ethyl 3-[8-[[4-methyl-5-[(3-methyl-4-oxophthalazin-1-yl)methyl]-1,2,4-triazol-3-yl]sulfanyl]octanoylamino]benzoate Chemical compound CCOC(=O)C1=CC(NC(=O)CCCCCCCSC2=NN=C(CC3=NN(C)C(=O)C4=CC=CC=C34)N2C)=CC=C1 GWNFQAKCJYEJEW-UHFFFAOYSA-N 0.000 description 1
- VICYTAYPKBLQFB-UHFFFAOYSA-N ethyl 3-bromo-2-oxopropanoate Chemical compound CCOC(=O)C(=O)CBr VICYTAYPKBLQFB-UHFFFAOYSA-N 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- OJCSPXHYDFONPU-UHFFFAOYSA-N etoac etoac Chemical compound CCOC(C)=O.CCOC(C)=O OJCSPXHYDFONPU-UHFFFAOYSA-N 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 208000019995 familial amyotrophic lateral sclerosis Diseases 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 210000003608 fece Anatomy 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 108010074605 gamma-Globulins Proteins 0.000 description 1
- 238000005227 gel permeation chromatography Methods 0.000 description 1
- ZTQSADJAYQOCDD-UHFFFAOYSA-N ginsenoside-Rd2 Natural products C1CC(C2(CCC3C(C)(C)C(OC4C(C(O)C(O)C(CO)O4)O)CCC3(C)C2CC2O)C)(C)C2C1C(C)(CCC=C(C)C)OC(C(C(O)C1O)O)OC1COC1OCC(O)C(O)C1O ZTQSADJAYQOCDD-UHFFFAOYSA-N 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 229960002449 glycine Drugs 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- JAXFJECJQZDFJS-XHEPKHHKSA-N gtpl8555 Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@H](B1O[C@@]2(C)[C@H]3C[C@H](C3(C)C)C[C@H]2O1)CCC1=CC=C(F)C=C1 JAXFJECJQZDFJS-XHEPKHHKSA-N 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 125000000262 haloalkenyl group Chemical group 0.000 description 1
- 125000004438 haloalkoxy group Chemical group 0.000 description 1
- 125000001188 haloalkyl group Chemical group 0.000 description 1
- 229960003878 haloperidol Drugs 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 239000008241 heterogeneous mixture Substances 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- ZTUJDPKOHPKRMO-UHFFFAOYSA-N hydron;2,2,2-trifluoroethanamine;chloride Chemical compound Cl.NCC(F)(F)F ZTUJDPKOHPKRMO-UHFFFAOYSA-N 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 125000002962 imidazol-1-yl group Chemical group [*]N1C([H])=NC([H])=C1[H] 0.000 description 1
- 208000026278 immune system disease Diseases 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 125000003387 indolinyl group Chemical group N1(CCC2=CC=CC=C12)* 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 230000031146 intracellular signal transduction Effects 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007919 intrasynovial administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- LRDFRRGEGBBSRN-UHFFFAOYSA-N isobutyronitrile Chemical compound CC(C)C#N LRDFRRGEGBBSRN-UHFFFAOYSA-N 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 201000005992 juvenile myelomonocytic leukemia Diseases 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 229940001447 lactate Drugs 0.000 description 1
- 229940099584 lactobionate Drugs 0.000 description 1
- JYTUSYBCFIZPBE-AMTLMPIISA-N lactobionic acid Chemical compound OC(=O)[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O JYTUSYBCFIZPBE-AMTLMPIISA-N 0.000 description 1
- 229940070765 laurate Drugs 0.000 description 1
- CFHGBZLNZZVTAY-UHFFFAOYSA-N lawesson's reagent Chemical compound C1=CC(OC)=CC=C1P1(=S)SP(=S)(C=2C=CC(OC)=CC=2)S1 CFHGBZLNZZVTAY-UHFFFAOYSA-N 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- DLEDOFVPSDKWEF-UHFFFAOYSA-N lithium butane Chemical compound [Li+].CCC[CH2-] DLEDOFVPSDKWEF-UHFFFAOYSA-N 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- RENRQMCACQEWFC-UGKGYDQZSA-N lnp023 Chemical compound C1([C@H]2N(CC=3C=4C=CNC=4C(C)=CC=3OC)CC[C@@H](C2)OCC)=CC=C(C(O)=O)C=C1 RENRQMCACQEWFC-UGKGYDQZSA-N 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 239000000391 magnesium silicate Substances 0.000 description 1
- 229910000386 magnesium trisilicate Inorganic materials 0.000 description 1
- 235000019793 magnesium trisilicate Nutrition 0.000 description 1
- 229940099273 magnesium trisilicate Drugs 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 229940049920 malate Drugs 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Natural products C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 1
- RMIODHQZRUFFFF-UHFFFAOYSA-N methoxyacetic acid Chemical compound COCC(O)=O RMIODHQZRUFFFF-UHFFFAOYSA-N 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 229940042472 mineral oil Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 239000002899 monoamine oxidase inhibitor Substances 0.000 description 1
- CQDGTJPVBWZJAZ-UHFFFAOYSA-N monoethyl carbonate Chemical compound CCOC(O)=O CQDGTJPVBWZJAZ-UHFFFAOYSA-N 0.000 description 1
- 229960005127 montelukast Drugs 0.000 description 1
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 1
- 229960004866 mycophenolate mofetil Drugs 0.000 description 1
- RTGDFNSFWBGLEC-SYZQJQIISA-N mycophenolate mofetil Chemical compound COC1=C(C)C=2COC(=O)C=2C(O)=C1C\C=C(/C)CCC(=O)OCCN1CCOCC1 RTGDFNSFWBGLEC-SYZQJQIISA-N 0.000 description 1
- MZRVEZGGRBJDDB-UHFFFAOYSA-N n-Butyllithium Substances [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 1
- UHXYXWIGCSBFBR-UHFFFAOYSA-N n-[3-(1h-pyrrolo[2,3-b]pyridin-4-yl)phenyl]acetamide Chemical compound CC(=O)NC1=CC=CC(C=2C=3C=CNC=3N=CC=2)=C1 UHXYXWIGCSBFBR-UHFFFAOYSA-N 0.000 description 1
- YGBMCLDVRUGXOV-UHFFFAOYSA-N n-[6-[6-chloro-5-[(4-fluorophenyl)sulfonylamino]pyridin-3-yl]-1,3-benzothiazol-2-yl]acetamide Chemical compound C1=C2SC(NC(=O)C)=NC2=CC=C1C(C=1)=CN=C(Cl)C=1NS(=O)(=O)C1=CC=C(F)C=C1 YGBMCLDVRUGXOV-UHFFFAOYSA-N 0.000 description 1
- XGYLUPDBTGIVMU-UHFFFAOYSA-N n-[[3-(1h-pyrrolo[2,3-b]pyridin-4-yl)phenyl]methyl]cyclopropanamine Chemical compound C=1C=CC(C=2C=3C=CNC=3N=CC=2)=CC=1CNC1CC1 XGYLUPDBTGIVMU-UHFFFAOYSA-N 0.000 description 1
- WOOWBQQQJXZGIE-UHFFFAOYSA-N n-ethyl-n-propan-2-ylpropan-2-amine Chemical compound CCN(C(C)C)C(C)C.CCN(C(C)C)C(C)C WOOWBQQQJXZGIE-UHFFFAOYSA-N 0.000 description 1
- OBKWWSIEURQEKL-UHFFFAOYSA-N n-methyl-1-[3-(1h-pyrrolo[2,3-b]pyridin-4-yl)phenyl]methanamine Chemical compound CNCC1=CC=CC(C=2C=3C=CNC=3N=CC=2)=C1 OBKWWSIEURQEKL-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-M naphthalene-2-sulfonate Chemical compound C1=CC=CC2=CC(S(=O)(=O)[O-])=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-M 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 210000003739 neck Anatomy 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- VWBWQOUWDOULQN-UHFFFAOYSA-N nmp n-methylpyrrolidone Chemical compound CN1CCCC1=O.CN1CCCC1=O VWBWQOUWDOULQN-UHFFFAOYSA-N 0.000 description 1
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- KVWDHTXUZHCGIO-UHFFFAOYSA-N olanzapine Chemical compound C1CN(C)CCN1C1=NC2=CC=CC=C2NC2=C1C=C(C)S2 KVWDHTXUZHCGIO-UHFFFAOYSA-N 0.000 description 1
- 229940049964 oleate Drugs 0.000 description 1
- PIDFDZJZLOTZTM-KHVQSSSXSA-N ombitasvir Chemical compound COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NC1=CC=C([C@H]2N([C@@H](CC2)C=2C=CC(NC(=O)[C@H]3N(CCC3)C(=O)[C@@H](NC(=O)OC)C(C)C)=CC=2)C=2C=CC(=CC=2)C(C)(C)C)C=C1 PIDFDZJZLOTZTM-KHVQSSSXSA-N 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 description 1
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 229960004851 pergolide Drugs 0.000 description 1
- YEHCICAEULNIGD-MZMPZRCHSA-N pergolide Chemical compound C1=CC([C@H]2C[C@@H](CSC)CN([C@@H]2C2)CCC)=C3C2=CNC3=C1 YEHCICAEULNIGD-MZMPZRCHSA-N 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L peroxydisulfate Chemical compound [O-]S(=O)(=O)OOS([O-])(=O)=O JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 150000003003 phosphines Chemical class 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 229940075930 picrate Drugs 0.000 description 1
- OXNIZHLAWKMVMX-UHFFFAOYSA-M picrate anion Chemical compound [O-]C1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1[N+]([O-])=O OXNIZHLAWKMVMX-UHFFFAOYSA-M 0.000 description 1
- 125000004194 piperazin-1-yl group Chemical group [H]N1C([H])([H])C([H])([H])N(*)C([H])([H])C1([H])[H] 0.000 description 1
- LWMPFIOTEAXAGV-UHFFFAOYSA-N piperidin-1-amine Chemical compound NN1CCCCC1 LWMPFIOTEAXAGV-UHFFFAOYSA-N 0.000 description 1
- 125000004482 piperidin-4-yl group Chemical group N1CCC(CC1)* 0.000 description 1
- 229950010765 pivalate Drugs 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 1
- 230000010118 platelet activation Effects 0.000 description 1
- 150000003057 platinum Chemical class 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 239000001818 polyoxyethylene sorbitan monostearate Substances 0.000 description 1
- 235000010989 polyoxyethylene sorbitan monostearate Nutrition 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229940113124 polysorbate 60 Drugs 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 239000004302 potassium sorbate Substances 0.000 description 1
- 235000010241 potassium sorbate Nutrition 0.000 description 1
- 229940069338 potassium sorbate Drugs 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- FASDKYOPVNHBLU-ZETCQYMHSA-N pramipexole Chemical compound C1[C@@H](NCCC)CCC2=C1SC(N)=N2 FASDKYOPVNHBLU-ZETCQYMHSA-N 0.000 description 1
- 229960003089 pramipexole Drugs 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000012746 preparative thin layer chromatography Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- QLNJFJADRCOGBJ-UHFFFAOYSA-N propionamide Chemical compound CCC(N)=O QLNJFJADRCOGBJ-UHFFFAOYSA-N 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 229950008679 protamine sulfate Drugs 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000002206 pyridazin-3-yl group Chemical group [H]C1=C([H])C([H])=C(*)N=N1 0.000 description 1
- 150000003222 pyridines Chemical class 0.000 description 1
- LJXQPZWIHJMPQQ-UHFFFAOYSA-N pyrimidin-2-amine Chemical group NC1=NC=CC=N1 LJXQPZWIHJMPQQ-UHFFFAOYSA-N 0.000 description 1
- 125000000246 pyrimidin-2-yl group Chemical group [H]C1=NC(*)=NC([H])=C1[H] 0.000 description 1
- 125000004527 pyrimidin-4-yl group Chemical group N1=CN=C(C=C1)* 0.000 description 1
- DOYOPBSXEIZLRE-UHFFFAOYSA-N pyrrole-3-carboxylic acid Natural products OC(=O)C=1C=CNC=1 DOYOPBSXEIZLRE-UHFFFAOYSA-N 0.000 description 1
- VONGYFFEWFJHNP-UHFFFAOYSA-N pyrrolecarboxylic acid methyl ester Natural products COC(=O)C1=CC=CN1 VONGYFFEWFJHNP-UHFFFAOYSA-N 0.000 description 1
- 150000003235 pyrrolidines Chemical class 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 229940038850 rebif Drugs 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 229940100618 rectal suppository Drugs 0.000 description 1
- 239000006215 rectal suppository Substances 0.000 description 1
- QEVHRUUCFGRFIF-MDEJGZGSSA-N reserpine Chemical compound O([C@H]1[C@@H]([C@H]([C@H]2C[C@@H]3C4=C(C5=CC=C(OC)C=C5N4)CCN3C[C@H]2C1)C(=O)OC)OC)C(=O)C1=CC(OC)=C(OC)C(OC)=C1 QEVHRUUCFGRFIF-MDEJGZGSSA-N 0.000 description 1
- 239000003340 retarding agent Substances 0.000 description 1
- 108010041788 rho-Associated Kinases Proteins 0.000 description 1
- 102000000568 rho-Associated Kinases Human genes 0.000 description 1
- 229960004181 riluzole Drugs 0.000 description 1
- 229940106887 risperdal Drugs 0.000 description 1
- RAPZEAPATHNIPO-UHFFFAOYSA-N risperidone Chemical compound FC1=CC=C2C(C3CCN(CC3)CCC=3C(=O)N4CCCCC4=NC=3C)=NOC2=C1 RAPZEAPATHNIPO-UHFFFAOYSA-N 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 229960002052 salbutamol Drugs 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000000582 semen Anatomy 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 229940035004 seroquel Drugs 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- UKLNMMHNWFDKNT-UHFFFAOYSA-M sodium chlorite Chemical compound [Na+].[O-]Cl=O UKLNMMHNWFDKNT-UHFFFAOYSA-M 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- AJPJDKMHJJGVTQ-UHFFFAOYSA-M sodium dihydrogen phosphate Chemical compound [Na+].OP(O)([O-])=O AJPJDKMHJJGVTQ-UHFFFAOYSA-M 0.000 description 1
- 235000009518 sodium iodide Nutrition 0.000 description 1
- KSAVQLQVUXSOCR-UHFFFAOYSA-M sodium lauroyl sarcosinate Chemical compound [Na+].CCCCCCCCCCCC(=O)N(C)CC([O-])=O KSAVQLQVUXSOCR-UHFFFAOYSA-M 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000008279 sol Substances 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000001587 sorbitan monostearate Substances 0.000 description 1
- 235000011076 sorbitan monostearate Nutrition 0.000 description 1
- 229940035048 sorbitan monostearate Drugs 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000012086 standard solution Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 239000003206 sterilizing agent Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 125000000547 substituted alkyl group Chemical group 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000001384 succinic acid Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 229960001967 tacrolimus Drugs 0.000 description 1
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 210000001138 tear Anatomy 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- MHXBHWLGRWOABW-UHFFFAOYSA-N tetradecyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCC MHXBHWLGRWOABW-UHFFFAOYSA-N 0.000 description 1
- WHRNULOCNSKMGB-UHFFFAOYSA-N tetrahydrofuran thf Chemical compound C1CCOC1.C1CCOC1 WHRNULOCNSKMGB-UHFFFAOYSA-N 0.000 description 1
- BSYVTEYKTMYBMK-UHFFFAOYSA-N tetrahydrofurfuryl alcohol Chemical compound OCC1CCCO1 BSYVTEYKTMYBMK-UHFFFAOYSA-N 0.000 description 1
- 125000003039 tetrahydroisoquinolinyl group Chemical group C1(NCCC2=CC=CC=C12)* 0.000 description 1
- 125000000147 tetrahydroquinolinyl group Chemical group N1(CCCC2=CC=CC=C12)* 0.000 description 1
- DDFYFBUWEBINLX-UHFFFAOYSA-M tetramethylammonium bromide Chemical compound [Br-].C[N+](C)(C)C DDFYFBUWEBINLX-UHFFFAOYSA-M 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 239000012049 topical pharmaceutical composition Substances 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- ZDPHROOEEOARMN-UHFFFAOYSA-N undecanoic acid Chemical compound CCCCCCCCCCC(O)=O ZDPHROOEEOARMN-UHFFFAOYSA-N 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical class CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000003871 white petrolatum Substances 0.000 description 1
- 210000002268 wool Anatomy 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229940039925 zyprexa Drugs 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/06—Antiabortive agents; Labour repressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/10—Drugs for genital or sexual disorders; Contraceptives for impotence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/14—Drugs for dermatological disorders for baldness or alopecia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
Definitions
- the present invention relates to compounds useful as inhibitors of Janus kinases (JAK) and Rho-associated coiled-coil forming protein serine/threonine kinases (ROCK).
- the invention also provides pharmaceutically acceptable compositions comprising the compounds of the invention and methods of using the compositions in the treatment of various disorders.
- JAK2 has been implicated in myeloproliferative disorders, which include polycythemia vera, essential thrombocythemia, chronic idiopathic myelofibrosis, myeloid metaplasia with myelofibrosis, chronic myeloid leukemia, chronic myelomonocytic leukemia, chronic eosinophilic leukemia, hypereosinophilic syndrome and systematic mast cell disease.
- R 1 , R 2 , R 3 , Z and Q are as defined below.
- substituent When more than one position in a given structure can be substituted with more than one substituent selected from a specified group, the substituent may be either the same or different at each position. [ 0109 ] As described herein, when the term “optionally substituted” precedes a list, said term refers to all of the subsequent substitutable groups in that list. If a substituent radical or structure is not identified or defined as “optionally substituted", the substituent radical or structure is unsubstituted. For example, if X is halogen; optionally substituted C 1-3 alkyl or phenyl; X may be either optionally substituted alkyl or optionally substituted phenyl.
- aliphatic or "aliphatic group”, as used herein, means a straight-chain (i.e., unbranched) or branched, substituted or unsubstituted hydrocarbon chain that is completely saturated or that contains one or more units of unsaturation. Unless otherwise specified, aliphatic groups contain 1-20 aliphatic carbon atoms. In some embodiments, aliphatic groups contain 1-10 aliphatic carbon atoms. In other embodiments, aliphatic groups contain 1-8 aliphatic carbon atoms. In still other embodiments, aliphatic groups contain 1-6 aliphatic carbon atoms, and In yet other embodiments, aliphatic groups contain 1-4 aliphatic carbon atoms.
- Suitable aliphatic groups include, but are not limited to, linear or branched, substituted or unsubstituted alkyl, alkenyl, or alkynyl groups. Further examples of aliphatic groups include methyl, ethyl, propyl, butyl, isopropyl, isobutyl, vinyl, and sec-butyl.
- cycloaliphatic refers to a monocyclic C 3 -C 8 hydrocarbon or bicyclic C 8 -C 12 hydrocarbon that is completely saturated or that contains one or more units of unsaturation, but which is not aromatic, that has a single point of attachment to the rest of the molecule, and wherein any individual ring in said bicyclic ring system has 3-7 members.
- Suitable cycloaliphatic groups include, but are not limited to, cycloalkyl, cycloalkenyl, and cycloalkynyl. Further examples of aliphatic groups include cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cycloheptyl, and cycloheptenyl.
- the "heterocycle”, “heterocyclyl”, “heterocycloaliphatic”, or “heterocyclic” group has three to fourteen ring members in which one or more ring members is a heteroatom independently selected from oxygen, sulfur, nitrogen, or phosphorus, and each ring in the system contains 3 to 7 ring members.
- heteroatom means one or more of oxygen, sulfur, nitrogen, phosphorus, or silicon, including any oxidized form of nitrogen, sulfur, phosphorus, or silicon, the quaternized form of any basic nitrogen, or a substitutable nitrogen of a heterocyclic ring, for example N (as in 3,4-dihydro-2H-pyrrolyl), NH (as in pyrrolidinyl) or NR + (as in N-substituted pyrrolidinyl).
- alkoxy refers to an alkyl group, as previously defined, attached to the principal carbon chain through an oxygen (“alkoxy”) or sulfur (“thioalkyl”) atom.
- haloalkyl means alkyl, alkenyl or alkoxy, as the case may be, substituted with one or more halogen atoms.
- halogen means F, Cl, Br, or I.
- aryl used alone or as part of a larger moiety as in “aralkyl”, “aralkoxy”, or “aryloxyalkyl”, refers to monocyclic, bicyclic, and tricyclic carbocyclic ring systems having a total of six to fourteen ring members, wherein at least one ring in the system is aromatic, wherein each ring in the system contains 3 to 7 ring members and that has a single point of attachment to the rest of the molecule.
- aryl may be used interchangeably with the term “aryl ring”. Examples of aryl rings would include phenyl, naphthyl, and anthracene.
- heteroaryl used alone or as part of a larger moiety as in “heteroaralkyl” or “heteroarylalkoxy”, refers to monocyclic, bicyclic, and tricyclic ring systems having a total of five to fourteen ring members, wherein at least one ring in the system is aromatic, at least one ring in the system contains one or more heteroatoms, wherein each ring in the system contains 3 to 7 ring members and that has a single point of attachment to the rest of the molec'ule.
- heteroaryl may be used interchangeably with the term “heteroaryl ring” or the term “heteroaromatic”.
- Further examples of heteroaryl rings include the following monocycles :
- an aryl (including aralkyl, aralkoxy, aryloxyalkyl and the like) or heteroaryl (including heteroaralkyl and heteroarylalkoxy and the like) group may contain one or more substituents. Suitable substituents on the unsaturated carbon atom of an aryl or heteroaryl group are selected from those listed in the definition of J Q , J R , J v , J u and J x below.
- Optional substituents on the aliphatic group or the phenyl ring of R + are selected from NH 2 , NH(C 1-4 aliphatic), N(C 1-4 aliphatic) 2 , halogen, C 1-4 aliphatic, OH, 0(C 1-4 aliphatic), NO 2 , CN, CO 2 H, CO 2 (C 1-4 aliphatic), O(halo C 1-4 aliphatic), or halo(C 1-4 aliphatic), wherein each of the foregoing C 1-4 aliphatic groups of R + is unsubstituted. [ 0125 ] As detailed above, in some embodiments, two independent occurrences of
- R 0 (or R + , or any other variable similarly defined herein), may be taken together with the atom(s) to which each variable is bound to form a 5-8-membered heterocyclyl, aryl, or heteroaryl ring or a 3-8-membered cycloalkyl ring.
- Exemplary rings that are formed when two independent occurrences of R 0 (or R + , or any other variable similarly defined herein) are taken together with the atom(s) to which each variable is bound include, but are not limited to the following: a) two independent occurrences of R 0 (or R + , or any other variable similarly defined herein) that are bound to the same atom and are taken together with that atom to form a ring, for example, N(R°) 2 , where both occurrences of R 0 are taken together with the nitrogen atom to form a piperidin-1-yl, piperazin-1-yl, or morpholin-4-yl group; and b) two independent occurrences of R 0 (or R + , or any other variable similarly defined herein) that are bound to different atoms and are taken together with both of those atoms to form a ring, for example where a phenyl group is substituted
- an alkyl or aliphatic chain can be optionally interrupted with another atom or group. This means that a methylene unit of the alkyl or aliphatic chain is optionally replaced with said other atom or group.
- Optional interruptions can occur both within the chain and at either end of the chain; i.e. both at the point of attachment and/or also at the terminal end.
- Two optional replacements can also be adjacent to each other within a chain so long as it results in a chemically stable compound. Unless otherwise specified, if the replacement or interruption occurs at the terminal end, the replacement atom is bound to an H on the terminal end. For example, if -CH 2 CH 2 CH 3 were optionally interrupted with -0-, the resulting compound could be -OCH 2 CH 3 , -CH 2 OCH 3 , or -CH 2 CH 2 OH.
- a bond drawn from a substituent to the center of one ring within a multiple-ring system represents substitution of the substituent at any substitutable position in any of the rings within the multiple ring system.
- Figure a represents possible substitution in any of the positions shown in Figure b.
- Figure a Figure b [0128] This also applies to multiple ring systems fused to optional ring systems (which would be represented by dotted lines).
- X is an optional substituent both for ring A and ring B.
- each substituent only represents substitution on the ring to which it is attached.
- Y is an optionally substituent for ring A only
- X is an optional substituent for ring B only.
- structures depicted herein are also meant to include all isomeric (e.g., enantiomeric, diastereomeric, and geometric (or conformational)) forms of the structure; for example, the R and S configurations for each asymmetric center, (Z) and (E) double bond isomers, and (Z) and (E) conformational isomers. Therefore, single stereochemical isomers as well as enantiomeric, diastereomeric, and geometric (or conformational) mixtures of the present compounds are within the scope of the invention.
- the present invention relates to a compound of formula I:
- Q is a 3-8 membered saturated, partially saturated, or unsaturated monocyclic ring having 0-3 heteroatoms selected from nitrogen, oxygen, or sulfur; or an 8-12 membered bicyclic ring having 0-6 heteroatoms selected from nitrogen, oxygen, or sulfur; wherein Q is optionally substituted with 1-10 occurrences of J Q ;
- R 1 is -(C 1-2 aliphatic) p -R 4 wherein each R 1 is optionally substituted with 1-3 occurrences of J;
- R 2 is -(C 1-2 aliphatic) d -R 5 wherein each R 1 is optionally substituted with 1-3 occurrences of J;
- R 3 is halogen, -CN, -NO 2 or -(U) m -X, wherein
- U is a C 1-6 aliphatic, wherein up to two methylene units are optionally and independently replaced by G u and wherein U is optionally substituted with 1-4 J u ;
- X is H, halogen, CN, NO 2 , S(O)R, SO 2 R, C 1-4 haloaliphatic, or a group selected from C 1-6 aliphatic, a C 3-1 O cycloaliphatic, C 6-1O aryl, 5-10 membered heteroaryl, 5-10 membered heterocyclyl; wherein said group is optionally substituted with 1-4 J x ;
- G u is -NH-, -NR-, -O-, -S-, -CO 2 -, -OC(O)-, -C(O)CO-, -C(O)-, -C(O)NH-,
- J Q is halogen, OCF 3 , -(Vn)-R", -(Vn)-CN, -(V n )-NO 2 , or -(V n )-(C 1-4 haloaliphatic), wherein J Q is not H;
- R" is H, or is an optionally substituted group selected from C 1-G aliphatic, C 3-10 cycloaliphatic, C 6-10 aryl, 5-10 membered heteroaryl, or 5-10 membered heterocyclyl; or two R" groups, on the same substituent or different substituents, together with the atom(s) to which each R" group is bound, form an optionally substituted 3-8 membered heterocyclyl; wherein each optionally substituted R" group is independently and optionally substituted with 1-6 occurrences of J R ;
- R is an optionally substituted group selected from C 1-6 aliphatic, C 3-1O cycloaliphatic, C 6- io aryl, 5-10 membered heteroaryl, or 5-10 membered heterocyclyl; or two R groups, on the same substituent or different substituents, together with the atom(s) to which each R group is bound, form an optionally substituted 3-8 membered heterocyclyl; wherein each R group is independently and optionally substituted with 1-4 occurrences of J ;
- J v , J u , J x , and J R are each independently selected from halogen, L, -(L n )-R ⁇ -(L n )-N(R) 2 , -(Ln)-SR', -(L n )-OR', -(L n )-(C 3-10 cycloaliphatic), -(Ln)-(C 6-10 aryl), -OU)-(S-IO membered heteroaryl), -(L n )-(5-10 membered heterocyclyl), oxo, C 1-4 haloalkoxy, C 1-4 haloalkyl, -(U)-NO 2 , -(Ln)-CN, -(Ln)-OH, -(L n )-CF 3 , -CO 2 R', -CO 2 H, -COR', -COH, -OC(O)R', or -NC(O
- R' is H or C 1-6 aliphatic; or two R' groups, together with the atom to which they are attached, optionally form a 3-6 membered cycloaliphatic or heterocyclyl, wherein said aliphatic, cycloaliphatic or heterocyclyl is optionally substituted with R*, - OR*, -SR*, -NO 2 , -CF 3 , -CN, -CO 2 R*, -COR*, OCOR*, NHCOR*, wherein R* is H or C 1-6 aliphatic;
- R 6 is selected from C 1-6 aliphatic, C 3-1O cycloaliphatic, C 6-1O aryl, 5-10 membered heteroaryl, or 5-10 membered heterocyclyl; or two R groups, on the same substituent or different substituents, together with the atom(s) to which each R 6 group is bound, form a 3-8 membered heterocyclyl;
- the invention provides a compound of formula I- a
- the invention provides a compound of formula I- b
- phenyl when R 1 and R 3 are H, R 2 is Me, and Z' is NH, then Q is not F ; when R 1 , R 2 and R 3 are H, and Z' is NH; then Q is not 3,5-difluoro phenyl, 4-amino phenyl, 4-nitro phenyl, 2-chloro-4-amino phenyl, 2-chloro-4-nitro phenyl, 3,4-
- R 1 is H, halogen, CN, NH 2 , NO 2 , CF 3 , CH 3 , NCH 3 , OCH 3 , or OH.
- R 1 is H, halogen, or CF 3 .
- R 1 is H.
- d is O.
- R 2 is H, halogen,
- R 2 is H, halogen, or CF 3 .
- R is H.
- both R 1 and R 2 are H.
- m is 0 and R 3 is H, halogen, -CN, -NO 2 or X.
- R 3 is H, halogen or C 1-3 aliphatic.
- R 3 is H or Cl.
- m is 1 and U is an optionally substituted C 1-3 aliphatic, wherein up to two methylene units are optionally replaced with 0-2 G u groups.
- G u is selected from -NH-, -NR-, -O-, -CO 2 -, -OC(O)- or -C(O)-.
- X is selected from H, C 1-6 aliphatic, halogen, CN, NO 2 , S(O) 0- 2 R, or C 1-4 haloalkyl.
- X is H, C 1-6 alkyl or halogen.
- X is H or Cl.
- R and R are H and R is H or Cl.
- Z is a bond and R 3 is selected from H, Cl, Br, F, -
- R 3 is selected from H, Cl, -Br, -CN, -COOH, -COOMe, - CONHR', -CON(Me) 2 , -CH 2 OH, -NO 2 , -NH 2 or an optionally substituted C 1 -C 4 aliphatic.
- R 3 is selected from Cl, -Br, -CN or an optionally substituted C 1 -C 4 aliphatic.
- R 3 is Cl.
- J u is selected from halogen, CN, NO 2 , C 1-
- haloalkyl OH, C 1-3 alkyl, -O-(C 1-2 alkyl), NH 2 , -NH-(C 1-2 alkyl), -N(C 1-2 alkyl) 2 , -O-(C 1-2 haloalkyl), or oxo.
- J x is selected from halogen, CN, NO 2 , C 1-
- haloalkyl OH, C 1-3 alkyl, -O-(C 1-2 alkyl), NH 2 , -NH-(C 1-2 alkyl), -N(Ci -2 alkyl) 2 , -O-(C 1-2 haloalkyl), or oxo.
- Q is a 5-10 membered aryl or heteroaryl ring optionally substituted with 1-5 J Q groups. In a further embodiment, Q is a 5-6 membered aryl or heteroaryl ring optionally substituted with 1-5 J Q groups. [0144 ] In another embodiment, Q is a 5-6 membered ring selected from:
- Q is a 6-membered ring selected from phenyl
- Q is phenyl (a), pyridyl (b), or pyrimidyl (c).
- Q is 2-pyridyl, 4-pyridyl, 2,6-pyrimidyl or phenyl.
- R and R are H.
- R 3 is H, halogen, or C 1-6 aliphatic.
- R 3 is H or Cl.
- Q is as described herein, Z is a bond. In a different embodiment, when Q is as described herein, Z is NH.
- Q is a 5-membered ring selected from pyrazolyl (h), thiophenyl (v), furanyl (u) or pyrrolyl (w).
- R 1 and R 2 are H.
- R 3 is H, halogen, or C 1-6 aliphatic.
- R 3 is H or Cl.
- Z is a bond. In a different embodiment, when Q is as described herein, Z is NH.
- Q is a 5-12 membered saturated or partially saturated monocyclic or bicyclic ring system optionally substituted with 1-5 J Q groups.
- Q is a 5-12 membered fully saturated or partially saturated monocyclic or bicyclic cycloaliphatic ring system.
- Q is a mono-unsaturated ring as represented by formula II:
- Q is a 5-6 membered fully saturated or partially saturated monocyclic cycloaliphatic ring system.
- Q is a 5-8 membered partially saturated heterocyclyl ring.
- Q is a 5-6 membered heterocyclyl containing 1-2 nitrogen atoms.
- Q contains 1 nitrogen atom.
- Q is the ring represented in formula III:
- Z is a bond or NH.
- R 1 and R 2 are H.
- R 3 is H, halogen, or C 1-6 aliphatic.
- R 3 is H or Cl.
- each occurrence of J Q is independently selected from R", -CH 2 R", halogen, CN, NO 2 , -COR", -COR 8 R", - N(R')R", -CH 2 N(R')R", -OR", -CH 2 OR", -SR", -CH 2 SR", -C(O)OR", -NR'COR", - NR 5 COR 8 R", -NR'COOR", -NR 5 COOR 8 R", -CON(R')R", -C0N(R')R 8 R", - SO 2 N(R')R", -SO 2 N(R')R 8 R", -C0N(R')R 8 N(R')R", -OR 8 OR", -OR 8 N(R')R", - NR'CH(R 8 )R", -NR'CH(R 8 )C(O)OR", -N(R')R 8 R
- each occurrence of J ⁇ is independently selected from R", -CH 2 R", halogen, -CN, -NO 2 , -N(R')R", -CH 2 N(R')R", -OR", -CH 2 OR", -SR", -CH 2 SR", -COOR", -NR'COR", -NR 5 COCH 2 R", -NR'CO(CH 2 ) 2 R", -NR'COOR", - C0N(R')R", -SO 2 N(R')R", -CONR'(CH 2 ) 2 N(R 5 )R", -CONR(CH 2 ) 3 N(R')R", - CONR'(CH 2 ) 4 N(R') R” , ⁇ O(CH 2 ) 2 OR", O(CH 2 ) 3 OR", O(CH 2 ) 4 OR", -O(CH 2 ) 2 N(R')R", - O(CH 2 ) 2 OR", O(CH 2 )
- R' or two occurrences of R' are taken together with the nitrogen atom to which they are bound to form an optionally substituted 3-10-membered monocyclic or bicyclic heterocyclic ring selected from:
- R 7 is independently R', -CH 2 R', halogen, , CH 2 CH, CN, NO 2 , -N(R') 2 , -CH 2 N(R') 2 , -OR', -CH 2 OR', -SR', -CH 2 SR', - COOR', -COR', -NR 9 COR', -NR 9 COOR', -CON(R') 2 , -SO 2 N(R') 2 , -NR 9 SO 2 R', - CONR 9 (CH 2 ) 2 N(R 9 )R', -CONR 9 (CH 2 ) 3 N(R 9 )R ⁇ -CONR 9 (CH 2 ) 4 N(R 9 )R', -O(CH 2 ) 2 OR', O(CH 2 ) 3 OR', O(CH 2 ) 4 OR', -O(CH 2 ) 2 N(R
- J Q is -V-R", -V-CN, -V-NO 2 , or -V-
- V is C 1-6 alkyl, wherein up to two methylene units are replaced by G v wherein G v is selected from -NH-, -NR-, -O-, -S-, -
- V is a Q ⁇ alltyl wherein zero methylene units are replaced by G v .
- V is CH 2 .
- V is substituted with 2 J v groups; wherein J v is C 1-3 alkyl or two J v groups, together with the methylene unit to which they are attached, form a 3-6 membered cycloalkyl ring.
- one methylene unit of V is replaced by G v .
- V is a Qalkyl, wherein one methylene unit is replaced by G v .
- G v is bonded directly to R".
- J v is selected from halogen, NH 2 , NO 2 , CN, OH,
- Q is 5-10 membered aryl or heteroaryl optionally substituted with 0-4 occurrences of J Q and J Q is halogen, OCF 3 , -(V n )-CN, -(V n )-N0 2 , or
- J Q is -(V n )-R" and V is a C 2-10 aliphatic, wherein up to three methylene units are replaced by G v , wherein G v is selected from -NH-, -NR-, -0-, -S-, -CO 2 -, -OC(O)-, -C(O)CO-, -C(O)-, -C(O)NH-, -C(O)NR-,
- R 5 is H, halogen, CN, NH 2 , NO 2 , CF 3 , C 1-3 aliphatic, cyclopropyl, NCH 3 , OCH 3 ,
- J v , J u , J x , and J R are each independently selected from halogen, L, -(L n )-R', -(L n )-N(R ) 2 , -(Ln)-SR', -(Ln)-OR', -(Ln)-(C 3 -I 0 cycloaliphatic), -(Ln)-(C 6-10 aryl), -(Ln)-(S-IO membered heteroaryl), -(L n )-(5-10 membered heterocyclyl), oxo, C 1-4 haloalkoxy, C ⁇ haloalkyl, -(Ln)-NO 2 , -(Ln)-CN, -(Ln)-OH, -(Ln)-CF 3 , -CO 2 R', -CO 2 H, -COR', -COH, -OC(O)R', or -NC(
- compositions of this invention may be administered in the form of suppositories for rectal administration.
- suppositories for rectal administration.
- suppositories can be prepared by mixing the agent with a suitable non-irritating excipient that is solid at room temperature but liquid at rectal temperature and therefore will melt in the rectum to release the drug.
- suitable non-irritating excipient include cocoa butter, beeswax and polyethylene glycols.
- compositions of this invention may also be administered topically, especially when the target of treatment includes areas or organs readily accessible by topical application, including diseases of the eye, the skin, or the lower intestinal tract. Suitable topical formulations are readily prepared for each of these areas or organs.
- the pharmaceutically acceptable compositions may be formulated in a suitable ointment containing the active component suspended or dissolved in one or more carriers.
- Carriers for topical administration of the compounds of this invention include, but are not limited to, mineral oil, liquid petrolatum, white petrolatum, propylene glycol, polyoxyethylene, polyoxypropylene compound, emulsifying wax and water.
- the pharmaceutically acceptable compositions can be formulated in a suitable lotion or cream containing the active components suspended or dissolved in one or more pharmaceutically acceptable carriers.
- the oral compositions can also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents.
- adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents.
- injectable preparations for example, sterile injectable aqueous or oleaginous suspensions may be formulated according to the known art using suitable dispersing or wetting agents and suspending agents.
- the sterile injectable preparation may also be a sterile injectable solution, suspension or emulsion in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3-butanediol.
- compositions for rectal or vaginal administration are preferably suppositories which can be prepared by mixing the compounds of this invention with suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
- suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active compound.
- Solid dosage forms for oral administration include capsules, tablets, pills, powders, and granules.
- the active compound is mixed with at least one inert, pharmaceutically acceptable excipient or carrier such as sodium citrate or dicalcium phosphate and/or a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol, and silicic acid, b) binders such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as glycerol, d) disintegrating agents such as agar—agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate, e) solution retarding agents such as paraffin, f) absorption accelerators such as quaternary ammonium compounds, g) wetting agents such as, for example, cetyl alcohol and glycerol monostearate, h) absorbents such as kaolin and bentonite clay
- Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like.
- the solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner. Examples of embedding compositions that can be used include polymeric substances and waxes. Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polethylene glycols and the like.
- the dosage forms may also comprise buffering agents. They may optionally contain opacifying agents and can also be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner.
- buffering agents include polymeric substances and waxes.
- Dosage forms for topical or transdermal administration of a compound of this invention include ointments, pastes, creams, lotions, gels, powders, solutions, sprays, inhalants or patches.
- the active component is admixed under sterile conditions with a pharmaceutically acceptable carrier and any needed preservatives or buffers as may be required.
- Ophthalmic formulation, ear drops, and eye drops are also contemplated as being within the scope of this invention.
- the present invention contemplates the use of transdermal patches, which have the added advantage of providing controlled delivery of a compound to the body.
- Such dosage forms can be made by dissolving or dispensing the compound in the proper medium.
- Absorption enhancers can also be used to increase the flux of the compound across the skin. The rate can be controlled by either providing a rate controlling membrane or by dispersing the compound in a polymer matrix or gel.
- agents the inhibitors of this invention may also be combined with include, without limitation: treatments for Alzheimer's Disease such as Aricept ® and Excelon ® ; treatments for Parkinson's Disease such as L-DOP A/carbidopa, entacapone, ropinrole, pramipexole, bromocriptine, pergolide, trihexephendyl, and amantadine; agents for treating Multiple Sclerosis (MS) such as beta interferon (e.g., Avonex ® and Rebif ® ), Copaxone ® , and mitoxantrone; treatments for asthma such as albuterol and Singulair ® ; agents for treating schizophrenia such as zyprexa, risperdal, seroquel, and haloperidol; anti-inflammatory agents such as corticosteroids, TNF blockers, IL-I RA, azathioprine, cyclophosphamide, and sulfasalazine;
- MS Multiple Sclerosis
- the invention comprises a method of inhibiting
- the invention comprises a method of inhibiting
- the invention comprises a method of treating or lessening the severity of a ROCK kinase-mediated condition or disease in a patient.
- ROCK-mediated condition or “disease”, as used herein, means any disease or other deleterious condition in which ROCK is known to play a role.
- ROCK- mediated condition or “disease” also means those diseases or conditions that are alleviated by treatment with a ROCK inhibitor.
- Such conditions include, without limitation, hypertension, angina pectoris, cerebrovascular contraction, asthma, peripheral circulation disorder, preterm labor, cancer, erectile dysfunction, atherosclerosis, spasm (cerebral vasospasm and coronary vasospasm), retinopathy (e.g., glaucoma), inflammatory disorders, autoimmune disorders, AE)S, osteoporosis, myocardial hypertrophy, ischemia/reperfusion-induced injury, and endothelial dysfunction.
- the present invention relates to a method for treating or lessening the severity of benign prostatic hyperplasia or diabetes.
- the method comprises the additional step of administering to said patient an additional therapeutic agent selected from a chemotherapeutic or anti-proliferative agent, an anti-inflammatory agent,, an immunomodulatory or immunosuppressive agent, a neurotrophic factor, an antipsychotic agent, an agent for treating cardiovascular disease, an agent for treating destructive bone disorders, an agent for treating liver disease, an anti-viral agent, an agent for treating blood disorders, an agent for treating diabetes, or an agent for treating immunodeficiency disorders, wherein said additional therapeutic agent is appropriate for the disease being treated and said additional therapeutic agent is administered together with said composition as a single dosage form or separately from said composition as part of a multiple dosage form.
- an additional therapeutic agent selected from a chemotherapeutic or anti-proliferative agent, an anti-inflammatory agent,, an immunomodulatory or immunosuppressive agent, a neurotrophic factor, an antipsychotic agent, an agent for treating cardiovascular disease, an agent for treating destructive bone disorders, an agent for treating liver disease, an anti-viral agent, an agent for treating blood disorders,
- said disease, condition, or disorder is hypertension, cerebral vasospasm, coronary vasospasm, bronchial asthma, preterm labor, erectile dysfunction, glaucoma, vascular smooth muscle cell proliferation, myocardial hypertrophy, malignoma, ischemia/reperfusion-induced injury, endothelial dysfunction, Crohn's Disease, colitis, neurite outgrowth, Raynaud's Disease, angina pectoris, Alzheimer's disease, benign prostatic hyperplasia, cardiac hypertrophy, perivascular fibrosis or atherosclerosis.
- the present invention relates to a method for treating or lessening the severity of a cancer.
- the present invention relates to a method for treating or lessening the severity of a cancer selected from brain (gliomas), breast, colon, head and neck, kidney, lung, liver, melanoma, ovarian, pancreatic, prostate, sarcoma, or thyroid.
- the present invention relates to a method for treating or lessening the severity of pancreatic, prostate, or ovarian cancer.
- the invention provides a method of inhibiting
- JAK-mediated disease means any disease or other deleterious condition in which a JAK family kinase, in particular JAK2 or JAK3, is known to play a role.
- Such conditions include, without limitation, immune responses such as allergic or type I hypersensitivity reactions, asthma, autoimmune diseases such as transplant rejection, graft versus host disease, rheumatoid arthritis, amyotrophic lateral sclerosis, and multiple sclerosis, neurodegenerative disorders such as familial amyotrophic lateral sclerosis
- FALS FALS
- solid and hematologic malignancies such as leukemias and lymphomas.
- the invention provides a method of treating or lessening the severity of a disease of condition selected from a proliferative disorder, a cardiac disorder, a neurodegenerative disorder, an autoimmune disorder, a condition associated with organ transplant, an inflammatory disorder, an immune disorder or an immunologically mediated disorder, comprising administering to said patient a compound or composition of the invention.
- the method comprises the additional step of administering to said patient an additional therapeutic agent selected from a chemotherapeutic or anti-proliferative agent, an anti-inflammatory agent, an immunomodulatory or immunosuppressive agent, a neurotrophic factor, an agent for treating cardiovascular disease, an agent for treating diabetes, or an agent for treating immunodeficiency disorders, wherein said additional therapeutic agent is appropriate for the disease being treated and said additional therapeutic agent is administered together with said composition as a single dosage form or separately from said composition as part of a multiple dosage form.
- an additional therapeutic agent selected from a chemotherapeutic or anti-proliferative agent, an anti-inflammatory agent, an immunomodulatory or immunosuppressive agent, a neurotrophic factor, an agent for treating cardiovascular disease, an agent for treating diabetes, or an agent for treating immunodeficiency disorders
- the disease or disorder is allergic or type I hypersensitivity reactions, asthma, diabetes, Alzheimer's disease, Huntington's disease, Parkinson's disease, AIDS-associated dementia, amyotrophic lateral sclerosis (ALS, Lou Gehrig's disease), multiple sclerosis (MS), schizophrenia, cardiomyocyte hypertrophy, reperfusion/ischemia, stroke, baldness, transplant rejection, graft versus host disease, rheumatoid arthritis, amyotrophic lateral sclerosis, and multiple sclerosis, and solid and hematologic malignancies such as leukemias and lymphomas.
- said disease or disorder is asthma.
- said disease or disorder is transplant rejection.
- a compound or composition of this invention may be used to treat a myeloproliferative disorder.
- the myeloproliferative disorder is polycythemia vera, essential thrombocythemia, or chronic idiopathic myelofibrosis.
- the myeloproliferative disorder is myeloid metaplasia with myelofibrosis, chronic myeloid leukemia (CML), chronic myelomonocytic leukemia, chronic eosinophilic leukemia, hypereosinophilic syndrome, systematic mast cell disease, atypical CML or juvenile myelomonocytic leukemia.
- an "effective amount" of the compound or pharmaceutically acceptable composition is that amount effective for treating or lessening the severity of one or more of the aforementioned disorders.
- the compounds and compositions, according to the method of the present invention may be administered using any amount and any route of administration effective for treating or lessening the severity of the disorder or disease. The exact amount required will vary from subject to subject, depending on the species, age, and general condition of the subject, the severity of the infection, the particular agent, its mode of administration, and the like.
- the methods of this invention comprise the additional step of separately administering to said patient an additional therapeutic agent.
- additional therapeutic agents When these additional therapeutic agents are administered separately they may be administered to the patient prior to, sequentially with or following administration of the compositions of this invention.
- the compounds of this invention or pharmaceutical compositions thereof may also be used for coating an implantable medical device, such as prostheses, artificial valves, vascular grafts,. stents and catheters.
- an implantable medical device such as prostheses, artificial valves, vascular grafts,. stents and catheters.
- Vascular stents for example, have been used to overcome restenosis (re-narrowing of the vessel wall after injury).
- patients using stents or other implantable devices risk clot formation or platelet activation. These unwanted effects may be prevented or mitigated by pre-coating the device with a pharmaceutically acceptable composition comprising a compound of this invention.
- Suitable coatings and the general preparation of coated implantable devices are described in US Patents 6,099,562; 5,886,026; and 5,304,121.
- the coatings are typically biocompatible polymeric materials such as a hydrogel polymer, polymethyldisiloxane, polycaprolactone, polyethylene glycol, polylactic acid, ethylene vinyl acetate, and mixtures thereof.
- the coatings may optionally be further covered by a suitable topcoat of fluorosilicone, polysaccarides, polyethylene glycol, phospholipids or combinations thereof to impart controlled release characteristics in the composition.
- Implantable devices coated with a compound of this invention are another embodiment of the present invention.
- the compounds may also be coated on implantable medical devices, such as beads, or co-formulated with a polymer or other molecule, to provide a "drug depot", thus permitting the drug to be released over a longer time period than administration of an aqueous solution of the drug.
- the compounds of this invention may be prepared in general by methods known to those skilled in the art for analogous compounds or by those methods depicted in the Examples below. See, e.g., the examples described in WO 2005/095400, which is herein incorporated by reference in its entirety.
- the solution was diluted with water (400 mL), cooled to O 0 C and filtered.
- the solid dried in a vac oven at 50 0 C to provide 4.92 g of brown solid which was used without purification.
- the brown solid was dissolved in DMF (40 mL) cooled to 0 °C and treated with NaH (60% in oil, 750 mg) followed by addition of toluenesulfonyl chloride (3.92 g, 20.5 mmol). Additional portions of NaH (60% in oil, 2 x 750 mg) were added at 1 h intervals. After 4 h, EtOAc was added and the reaction was quenched with sat'd NaHCO 3 .
- the title compound (116) was prepared using method B to afford 0.7 mg as an off-white solid.
- the container was sealed and the reaction mixture was exposed to microwave irradiation for 30 min at 160 0 C.
- the resulting mixture was diluted in ethyl acetate, washed with water and dried over anhydrous Na 2 SO 4 .
- the crude material was purified by flash chromatography on silica gel, eluting with hexanes/ethyl acetate mixtures (90:10 to 60:40).
- the title compound J was isolated as a pale yellow solid (2.1 g, 50%).
- the reaction mixture was quenched with a solution of aqueous HCl 2M (10 mL) and stirred at room temperature for 1 h.
- the organic layer was then separated, washed with aqueous HCl 0.5 M, dried over anhydrous Na 2 SO 4 and the solvent removed under a stream of nitrogen.
- a second crop of desired product was obtained after neutralizing the aqueous layer with saturated solution of NaHCO 3 and extracting with ethyl acetate, followed by drying over anhydrous Na 2 SO 4 and removing the organic solvent under a stream of nitrogen.
- the title compound (M) was isolated as a white solid (1.27 g, 64%).
- the crude material was dissolved in DMSO and purified by reversed phase ⁇ PLC, eluting with Acetonitrile/water/l%TFA mixtures, yielding iV-(6-(l-tosyl-(lH- pyrrolo[2,3-b]pyridine ⁇ 4-yl)pyridin-2-yl)acetamine (R).
- R was dissolved in methanol and treated with NaOMe 0.5 M(1.5 mL). The resulting mixture was warmed at 60 0 C for 30 min.
- the crude was diluted in ethyl acetate, washed with saturated aqueous NaHCO 3 , dried over anhydrous Na 2 SO 4 , followed by removal of the solvent under reduced pressure.
- the crude oil was dissolved in DMSO (2 mL) and purified by reversed phase HPLC, eluting with acetonitrile/water/l%TFA mixtures.
- the title compound (98) was isolated after removing the solvents using a liophilizer (vacuum and heat lead to decomposition of the sample). (2.4 mg, 8%).
- the reaction was quenched with a saturated solution of NH 4 Cl, followed by aqueous NaHCO 3 .
- the crude reaction was diluted in diethyl ether, washed with brine, dried over anhydrous Na 2 SO 4 and the solvent was then removed under reduced pressure.
- the resulting material was purified by flash chromatography on silica gel, eluting with mixtures of dichloromethane/hexanes (2:1). The title compound U was isolated as a white solid (1.1 g, 51%).
- VV (0.075 g, 0.2 mmol) was dissolved in DMF under nitrogen.
- DIEA DIEA
- Method C was used in the case of aliphatic and liquid amines (Scheme 3), while Pd-catalyzed coupling was used for solid and aromatic amines (Scheme 4).
- the amide part on the indole nitrogen was hydrolysed selectively in presence of the amide on the piperidine /pyrrolidine nitrogen. [0385 ] Due to this formation of disubstituted products, removal of the tosyl group after reaction with acid chlorides was preferred.
- Acids could be coupled after Boc deprotection in the presence of tosyl group on the indole nitrogen but attemps to remove this protecting group were unsuccessful.
- the newly introduced amides were hydrolized. Products from 3,3,3- trifluoropropionic acid and cyanoacetic acid were obtained after deprotection of tosyl and Boc protecting groups in this order and then using a coupling reagent.
- Method C To D (20 mg, 0.086 mmol) was added amine (0.5 ml) in a pressure tube flushed with N 2 . The mixture was heated to 23O 0 C (bath temperature) for 24 h. The mixture was concentrated. Preparative HPLC provided 5-chloro-iV- cyclopentyl-lH-pyrrolo[2,3-b]pyridin-4-amine (7.4 mg) as the TFA salt. [0390] Method D: Pd(OAc) 2 (3.9 mg, 0.015mmol) and ( ⁇ )-BINAP (20 mg,
- Boc-NH- R compounds were treated with a standard solution 6N of HCl in IPA (1 mmol of compound in 4 mL), stirred 2 h at room temperature to remove Boc and isolated after the solvent was removed in vacuo.
- IPA a standard solution 6N of HCl in IPA (1 mmol of compound in 4 mL)
- triethylamine 3 eq
- the mixture was stirred for 10 min at room temperature. Water was added and the phases were separated. The organic layer was dried over sodium sulfate and the solvent was removed in vacuo.
- the amines were used without purification.
- R' compounds were obtained in 90% yield from E with JV-Boc-3- aminopyrrolidine or iV-Boc-3-aminopiperidme using method D. If needed, compound R' could be separated from BDSfAP impurities by chromatography eluting with CH 2 Cl 2 : EtOAc 20:1.
- a compound of formula S' (60 mg) was dissolved in a 2: 1 THF:MeOH mixture (4 ml) and 6N aq KOH solution (0.8 ml) was added. After 1 h at room temperature, solvents were evaporated and the mixture taken up in ethyl acetate, washed with brine, dried (Na 2 SO 4 ), filtrated and concentrated. The product could be purified by column chromatography (heptane:EtOAc 1:2 mixture). The product was then dissolved in dichloromethane (3 ml) and 5 N HCl in 2-propanol (0.7 ml) was added. After 1 h at room temperature the mixture was evaporated to dryness to give a white salt.
- the crude compound K was purified by column chromatography (silica gel 100-200 mesh, MeOHZCHCl 3 ⁇ l%MeOH/CHCl 3 ).
- the Boc group from K" may be removed by standard procedures to obtain L".
- Example 2 NMR and Mass Spectrometry of Compounds
- Analytical data for certain compounds of the present invention was collected and recorded.
- proton NMR was collected using a Bruker AMX 500 instrument and appropriate solvent.
- the LC/MS method used a Hypersil BDS Cl 8 5 micron 2.1 x 50 mm column with a flow rate of l.Oml/min with an appropriate gradient.
- Mass spectrometer samples were analyzed on a MicroMass ZQ or Quattro II mass spectrometer operated in single MS mode with electrospray ionization. Samples were introduced into the mass spectrometer using flow injection (FIA) or chromatography. Mobile phase for all mass spectrometer analysis consisted of acetonitrile-water mixtures or TFA in some instances.
- Table 3 below depicts exemplary LC mass spectral data (LC/MS), retention time (RT) and 1 H-NMR data for certain compounds of the present invention, wherein compound numbers in Table 3 correspond to the compounds depicted in Table 1 (empty cells indicate that the test was not performed): Table 3
- Table 4 below depicts exemplary LC/MS, RT and 1 H-NMR data for certain compounds of the present invention, wherein compound numbers in Table 4 correspond to the compounds depicted in Table 2 (empty cells indicate that the test was not performed). For the compounds in Table 4, proton NMR was collected using as indicated. Table 4
- 1.5 ⁇ l of DMSO stock containing serial dilutions of the compound of the present invention (concentrations ranging from 10 ⁇ M to 2.6nM) was placed in a 96 well plate.
- 50 ⁇ l of Solution 1 100 mM HEPES (pH 7.5), 10 mM MgCl 2 , 26 mM [ ⁇ - 33 P] ATP was added to the plate.
- the reaction was initiated by addition of 50 ⁇ l of Solution 2 (100 mM HEPES (pH 7.5), 10 mM MgCl 2 , 4 mM DTT, 54 mM MBP and 10 nM ROCK).
- Tables 5 and 6 depict enzyme inhibition data (Ki) for certain exemplary compounds.
- Compound numbers in Table 5 correspond to those compounds depicted in Table 1, while compound numbers in Table 6 correspond to those compounds depicted in Table 2.
- “A” represents a K 1 of less than 0.5 ⁇ M
- “B” represents a Kj of between 0.5 and 5.0 ⁇ M
- “C” represents a Kj greater than 5.0 ⁇ M for the indicated enzyme (for Table 6, "C” represents a Ki greater than 4.0 ⁇ M for ROCK I).
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Immunology (AREA)
- Physical Education & Sports Medicine (AREA)
- Pulmonology (AREA)
- Heart & Thoracic Surgery (AREA)
- Diabetes (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Oncology (AREA)
- Rheumatology (AREA)
- Virology (AREA)
- Cardiology (AREA)
- Endocrinology (AREA)
- Reproductive Health (AREA)
- Dermatology (AREA)
- Communicable Diseases (AREA)
- Gynecology & Obstetrics (AREA)
- Hematology (AREA)
- Psychiatry (AREA)
- Psychology (AREA)
- Transplantation (AREA)
- Pain & Pain Management (AREA)
- Urology & Nephrology (AREA)
- Molecular Biology (AREA)
Abstract
Description
Claims
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES06770824T ES2380795T3 (en) | 2005-05-20 | 2006-05-22 | Pyrrolopyridines useful as protein kinase inhibitors |
| AU2006251623A AU2006251623A1 (en) | 2005-05-20 | 2006-05-22 | Pyrrolopyridines useful as inhibitors of protein kinase |
| JP2008512591A JP2008545660A (en) | 2005-05-20 | 2006-05-22 | Pyrropyridine compounds useful as protein kinase inhibitors |
| MX2007014619A MX2007014619A (en) | 2005-05-20 | 2006-05-22 | Pyrrolopyridines useful as inhibitors of protein kinase. |
| CN2006800263918A CN101228161B (en) | 2005-05-20 | 2006-05-22 | Pyrrolopyridines useful as inhibitors of protein kinase |
| NZ564065A NZ564065A (en) | 2005-05-20 | 2006-05-22 | Pyrrolopyridines useful as inhibitors of protein kinase |
| AT06770824T ATE540948T1 (en) | 2005-05-20 | 2006-05-22 | PYRROLOPYRIDINES AS PROTEIN KINASE INHIBITORS |
| EP06770824A EP1881983B1 (en) | 2005-05-20 | 2006-05-22 | Pyrrolopyridines useful as inhibitors of protein kinase |
| CA002609126A CA2609126A1 (en) | 2005-05-20 | 2006-05-22 | Pyrrolopyridines useful as inhibitors of protein kinase |
| IL187438A IL187438A0 (en) | 2005-05-20 | 2007-11-18 | Pyrrolopyridine derivatives and pharmaceutical compositions containing the same |
| NO20076502A NO20076502L (en) | 2005-05-20 | 2007-12-18 | Pyrrolopyridines useful as inhibitors of protein kinase |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US68355405P | 2005-05-20 | 2005-05-20 | |
| US60/683,554 | 2005-05-20 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2006127587A1 true WO2006127587A1 (en) | 2006-11-30 |
Family
ID=36940144
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2006/019711 WO2006127587A1 (en) | 2005-05-20 | 2006-05-22 | Pyrrolopyridines useful as inhibitors of protein kinase |
Country Status (16)
| Country | Link |
|---|---|
| US (1) | US8921376B2 (en) |
| EP (3) | EP2354139A1 (en) |
| JP (3) | JP2008545660A (en) |
| KR (1) | KR20080016659A (en) |
| CN (1) | CN101228161B (en) |
| AT (1) | ATE540948T1 (en) |
| AU (1) | AU2006251623A1 (en) |
| CA (1) | CA2609126A1 (en) |
| ES (1) | ES2380795T3 (en) |
| IL (1) | IL187438A0 (en) |
| MX (1) | MX2007014619A (en) |
| NO (1) | NO20076502L (en) |
| NZ (1) | NZ564065A (en) |
| RU (2) | RU2435769C2 (en) |
| WO (1) | WO2006127587A1 (en) |
| ZA (1) | ZA200710379B (en) |
Cited By (123)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007070514A1 (en) * | 2005-12-13 | 2007-06-21 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors |
| WO2007077949A1 (en) * | 2005-12-28 | 2007-07-12 | Astellas Pharma Inc. | Heterocyclic janus kinase 3 inhibitors |
| WO2007082808A3 (en) * | 2006-01-18 | 2007-09-13 | Hoffmann La Roche | Thiazoles as 11 beta-hsd1 inhibitors |
| WO2007084557A3 (en) * | 2006-01-17 | 2007-09-27 | Vertex Pharma | Azaindoles useful as inhibitors of janus kinases |
| WO2007084667A3 (en) * | 2006-01-19 | 2007-12-06 | Osi Pharmaceutical Inc | Fused heterobicyclic kinase inhibitors |
| EP1910358A2 (en) * | 2005-07-14 | 2008-04-16 | Astellas Pharma Inc. | Heterocyclic janus kinase 3 inhibitors |
| WO2008084861A1 (en) | 2007-01-12 | 2008-07-17 | Astellas Pharma Inc. | Condensed pyridine compound |
| WO2007136790A3 (en) * | 2006-05-18 | 2008-11-27 | Mannkind Corp | Intracellular kinase inhibitors |
| WO2008116139A3 (en) * | 2007-03-22 | 2008-12-18 | Vertex Pharma | N-heterocyclic compounds useful as inhibitors of janus kinases |
| US7498342B2 (en) | 2004-06-17 | 2009-03-03 | Plexxikon, Inc. | Compounds modulating c-kit activity |
| US7504509B2 (en) | 2003-12-19 | 2009-03-17 | Plexxikon, Inc. | Compounds and methods for development of Ret modulators |
| WO2008145688A3 (en) * | 2007-06-01 | 2009-03-26 | Glaxo Group Ltd | Pyrrolopyridine compounds, process for their preparation, and their use as medicaments |
| WO2009112473A1 (en) * | 2008-03-12 | 2009-09-17 | Glaxo Group Limited | Novel compounds |
| JP2010509356A (en) * | 2006-11-10 | 2010-03-25 | ブリストル−マイヤーズ スクイブ カンパニー | Novel kinase inhibitor |
| DE102008052943A1 (en) | 2008-10-23 | 2010-04-29 | Merck Patent Gmbh | azaindole derivatives |
| JP2010513319A (en) * | 2006-12-18 | 2010-04-30 | インスパイアー ファーマシューティカルズ,インコーポレイティド | Cytoskeletal active RHO kinase inhibitor compounds, compositions and uses |
| US7834022B2 (en) | 2007-06-13 | 2010-11-16 | Incyte Corporation | Metabolites of the Janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US7846941B2 (en) | 2005-05-17 | 2010-12-07 | Plexxikon, Inc. | Compounds modulating c-kit and c-fms activity and uses therefor |
| US7863289B2 (en) | 2006-12-21 | 2011-01-04 | Plexxikon, Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US7863288B2 (en) | 2005-06-22 | 2011-01-04 | Plexxikon, Inc. | Compounds and methods for kinase modulation, and indications therefor |
| WO2011003418A1 (en) | 2009-07-08 | 2011-01-13 | Leo Pharma A/S | Heterocyclic compounds as jak receptor and protein tyrosine kinase inhibitors |
| US7872018B2 (en) | 2006-12-21 | 2011-01-18 | Plexxikon, Inc. | Compounds and methods for kinase modulation, and indications therefor |
| WO2011017142A1 (en) * | 2009-08-06 | 2011-02-10 | Merck Patent Gmbh | Novel bicyclic urea compounds |
| WO2011017009A1 (en) | 2009-08-07 | 2011-02-10 | Merck Patent Gmbh | Novel azaheterocyclic compounds |
| US7893075B2 (en) | 2006-11-22 | 2011-02-22 | Plexxikon, Inc. | Compounds modulating c-fms and/or c-kit activity and uses therefor |
| EP1968568A4 (en) * | 2005-12-22 | 2011-04-13 | Glaxosmithkline Llc | INHIBITORS OF Akt ACTIVITY |
| EP2303021A4 (en) * | 2008-06-16 | 2012-03-14 | Univ Tennessee Res Foundation | COMPOUNDS FOR TREATING CANCER |
| US8158616B2 (en) | 2008-03-11 | 2012-04-17 | Incyte Corporation | Azetidine and cyclobutane derivatives as JAK inhibitors |
| US8188281B2 (en) | 2004-03-30 | 2012-05-29 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of JAK and other protein kinases |
| CN102510865A (en) * | 2009-07-31 | 2012-06-20 | 日本烟草产业株式会社 | Nitrogen-containing spiro-ring compound and medicinal use of same |
| WO2012093169A1 (en) | 2011-01-07 | 2012-07-12 | Leo Pharma A/S | Novel sulfamide piperazine derivatives as protein tyrosine kinase inhibitors and pharmaceutical use thereof |
| US8247421B2 (en) | 2006-12-21 | 2012-08-21 | Vertex Pharmaceuticals Incorporated | 5-cyano-4-(pyrrolo [2,3B] pyridine-3-yl)-pyrimidine derivatives useful as protein kinase inhibitors |
| US8268858B2 (en) | 2006-12-21 | 2012-09-18 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| JP2012527481A (en) * | 2009-05-22 | 2012-11-08 | インサイト・コーポレイション | 3- [4- (7H-Pyrrolo [2,3-D] pyrimidin-4-yl) -1H-pyrazol-1-yl] octane- or heptane-nitrile as JAK inhibitors |
| US8513414B2 (en) | 2011-07-05 | 2013-08-20 | Vertex Pharmaceuticals Incorporated | Processes and intermediates for producing azaindoles |
| US8513270B2 (en) | 2006-12-22 | 2013-08-20 | Incyte Corporation | Substituted heterocycles as Janus kinase inhibitors |
| WO2013132270A1 (en) * | 2012-03-08 | 2013-09-12 | Karus Therapeutics Limited | Phosphoinositide 3-kinase inhibitors |
| WO2013148748A1 (en) * | 2012-03-29 | 2013-10-03 | Francis Xavier Tavares | Lactam kinase inhibitors |
| US8563541B2 (en) | 2005-09-22 | 2013-10-22 | Incyte Corporation | Azepine inhibitors of Janus kinases |
| US8653262B2 (en) | 2007-05-31 | 2014-02-18 | Boehringer Ingelheim International Gmbh | CCR2 receptor antagonists and uses thereof |
| US8691807B2 (en) | 2011-06-20 | 2014-04-08 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US8722693B2 (en) | 2007-06-13 | 2014-05-13 | Incyte Corporation | Salts of the Janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US8741920B2 (en) | 2009-08-03 | 2014-06-03 | Hoffmann-La Roche, Inc. | Process for the manufacture of pharmaceutically active compounds |
| FR3001219A1 (en) * | 2013-01-22 | 2014-07-25 | Centre Nat Rech Scient | KINASE INHIBITORS |
| US8822513B2 (en) | 2010-03-01 | 2014-09-02 | Gtx, Inc. | Compounds for treatment of cancer |
| US8829007B2 (en) | 2009-06-17 | 2014-09-09 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US8835440B2 (en) | 2008-12-19 | 2014-09-16 | Boehringer Ingelheim International Gmbh | Cyclic pyrimidin-4-carboxamides as CCR2 receptor antagonists for treatment of inflammation, asthma and COPD |
| US8841313B2 (en) | 2010-05-17 | 2014-09-23 | Boehringer Ingelheim International Gmbh | CCR2 antagonists and uses thereof |
| US8865735B2 (en) | 2011-02-21 | 2014-10-21 | Hoffman-La Roche Inc. | Solid forms of a pharmaceutically active substance |
| US8871774B2 (en) | 2010-12-16 | 2014-10-28 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US8877745B2 (en) | 2010-05-12 | 2014-11-04 | Boehringer Ingelheim International Gmbh | CCR2 receptor antagonists, method for producing the same, and use thereof as medicaments |
| US8933085B2 (en) | 2010-11-19 | 2015-01-13 | Incyte Corporation | Cyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors |
| US8946218B2 (en) | 2010-05-12 | 2015-02-03 | Boehringer Ingelheim International Gmbh | CCR2 receptor antagonists, method for producing the same, and use thereof as medicaments |
| US8962656B2 (en) | 2010-06-01 | 2015-02-24 | Boehringer Ingelheim International Gmbh | CCR2 antagonists |
| US8987443B2 (en) | 2013-03-06 | 2015-03-24 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US9018212B2 (en) | 2010-05-25 | 2015-04-28 | Boehringer Ingelheim International Gmbh | Pyridazine carboxamides as CCR2 receptor antagonists |
| US9029408B2 (en) | 2008-06-16 | 2015-05-12 | Gtx, Inc. | Compounds for treatment of cancer |
| US9034884B2 (en) | 2010-11-19 | 2015-05-19 | Incyte Corporation | Heterocyclic-substituted pyrrolopyridines and pyrrolopyrimidines as JAK inhibitors |
| US9051319B2 (en) | 2011-08-01 | 2015-06-09 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US9096593B2 (en) | 2009-11-06 | 2015-08-04 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US9108958B2 (en) | 2011-07-15 | 2015-08-18 | Boehringer Ingelheim International Gmbh | Selective CCR2 antagonists |
| CN104926810A (en) * | 2008-05-13 | 2015-09-23 | 阵列生物制药公司 | Pyrrolopyridines As Kinase Inhibitors |
| US9150570B2 (en) | 2012-05-31 | 2015-10-06 | Plexxikon Inc. | Synthesis of heterocyclic compounds |
| US9193733B2 (en) | 2012-05-18 | 2015-11-24 | Incyte Holdings Corporation | Piperidinylcyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors |
| US9249145B2 (en) | 2009-09-01 | 2016-02-02 | Incyte Holdings Corporation | Heterocyclic derivatives of pyrazol-4-yl-pyrrolo[2,3-d]pyrimidines as janus kinase inhibitors |
| WO2016028971A1 (en) * | 2014-08-21 | 2016-02-25 | Bristol-Myers Squibb Company | Tied-back benzamide derivatives as potent rock inhibitors |
| US9334274B2 (en) | 2009-05-22 | 2016-05-10 | Incyte Holdings Corporation | N-(hetero)aryl-pyrrolidine derivatives of pyrazol-4-yl-pyrrolo[2,3-d]pyrimidines and pyrrol-3-yl-pyrrolo[2,3-d]pyrimidines as janus kinase inhibitors |
| US9334242B2 (en) | 2008-06-16 | 2016-05-10 | Gtx, Inc. | Compounds for treatment of cancer |
| US9359358B2 (en) | 2011-08-18 | 2016-06-07 | Incyte Holdings Corporation | Cyclohexyl azetidine derivatives as JAK inhibitors |
| US9358229B2 (en) | 2011-08-10 | 2016-06-07 | Novartis Pharma Ag | JAK PI3K/mTOR combination therapy |
| KR20160102568A (en) | 2014-02-04 | 2016-08-30 | 다이호야쿠힌고교 가부시키가이샤 | Azaindole derivative |
| US9447089B2 (en) | 2009-04-03 | 2016-09-20 | Plexxikon Inc. | Compositions and uses thereof |
| US9447049B2 (en) | 2010-03-01 | 2016-09-20 | University Of Tennessee Research Foundation | Compounds for treatment of cancer |
| US9464092B2 (en) | 2013-03-15 | 2016-10-11 | G1 Therapeutics, Inc. | Transient protection of normal cells during chemotherapy |
| US9464088B2 (en) | 2010-03-10 | 2016-10-11 | Incyte Holdings Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| US9469640B2 (en) | 2007-07-17 | 2016-10-18 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US9487521B2 (en) | 2011-09-07 | 2016-11-08 | Incyte Holdings Corporation | Processes and intermediates for making a JAK inhibitor |
| US9498467B2 (en) | 2014-05-30 | 2016-11-22 | Incyte Corporation | Treatment of chronic neutrophilic leukemia (CNL) and atypical chronic myeloid leukemia (aCML) by inhibitors of JAK1 |
| US9512161B2 (en) | 2009-10-09 | 2016-12-06 | Incyte Corporation | Hydroxyl, keto, and glucuronide derivatives of 3-(4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US9580442B2 (en) | 2009-08-20 | 2017-02-28 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| AU2015201850B2 (en) * | 2005-12-13 | 2017-03-02 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| US9624213B2 (en) | 2011-02-07 | 2017-04-18 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US9655854B2 (en) | 2013-08-07 | 2017-05-23 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| US9670222B2 (en) | 2009-12-17 | 2017-06-06 | Centrexion Therapeutics Corporation | CCR2 receptor antagonists and uses thereof |
| US9676765B2 (en) | 2012-11-07 | 2017-06-13 | Karus Therapeutics Limited | Histone deacetylase inhibitors and their use in therapy |
| US9717735B2 (en) | 2014-04-17 | 2017-08-01 | G1 Therapeutics, Inc. | Tricyclic lactams for use in HSPC-sparing treatments for RB-positive abnormal cellular proliferation |
| US9771361B2 (en) | 2013-11-13 | 2017-09-26 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US9862685B2 (en) | 2013-05-10 | 2018-01-09 | Karus Therapeutics Limited | Histone deacetylase inhibitors |
| US9981987B2 (en) | 2014-02-12 | 2018-05-29 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US9993480B2 (en) | 2011-02-18 | 2018-06-12 | Novartis Pharma Ag | mTOR/JAK inhibitor combination therapy |
| US10023569B2 (en) | 2013-11-13 | 2018-07-17 | Vertex Pharmaceuticals Incorporated | Methods of preparing inhibitors of influenza viruses replication |
| US10059709B2 (en) | 2014-03-28 | 2018-08-28 | Elanco Tiergesundheit Ag | Compounds |
| US10166191B2 (en) | 2012-11-15 | 2019-01-01 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| US10213428B2 (en) | 2015-07-02 | 2019-02-26 | Centrexion Therapeutics Corporation | (4-((3R,4R)-3-methoxytetrahydro-pyran-4-ylamino)piperidin-1-yl)(5-methyl-6-(((2R,6S)-6-(p-tolyl)tetrahydro-2H-pyran-2-yl)methylamino)pyrimidin-4-yl)methanone citrate |
| US10231969B2 (en) | 2014-09-12 | 2019-03-19 | GI Therapeutics, Inc. | Anti-neoplastic combinations and dosing regimens using CDK4/6 inhibitor compounds to treat RB-positive tumors |
| WO2019057103A1 (en) * | 2017-09-21 | 2019-03-28 | 上海华汇拓医药科技有限公司 | Jak inhibitor, preparation method therefor, and application in the field of medicine |
| US10273233B2 (en) | 2015-05-13 | 2019-04-30 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US10323023B2 (en) | 2017-06-30 | 2019-06-18 | Beijing Tide Pharmaceutical Co., Ltd. | Rho-associated protein kinase inhibitor, pharmaceutical composition comprising the same, as well as preparation method and use thereof |
| US10329282B2 (en) | 2017-06-30 | 2019-06-25 | Beijing Tide Pharmaceutical Co., Ltd. | Rho-associated protein kinase inhibitor, pharmaceutical composition comprising the same, as well as preparation method and use thereof |
| US10377764B2 (en) | 2015-08-19 | 2019-08-13 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US10407435B2 (en) | 2014-10-29 | 2019-09-10 | Karus Therapeutics Limited | Diheteroaryl histone deacetylase inhibitors and their use in therapy |
| US10413547B2 (en) | 2014-09-12 | 2019-09-17 | G1 Therapeutics, Inc. | Treatment of Rb-negative tumors using topoisomerase with cyclin dependent kinase 4/6 inhibitors |
| WO2019179525A1 (en) * | 2018-03-23 | 2019-09-26 | Fochon Pharmaceuticals, Ltd. | Deuterated compounds as rock inhibitors |
| US10442815B2 (en) | 2015-08-19 | 2019-10-15 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US10533004B2 (en) | 2015-05-13 | 2020-01-14 | Vertex Pharmaceuticals Incorporated | Methods of preparing inhibitors of influenza viruses replication |
| US10533003B2 (en) | 2014-10-29 | 2020-01-14 | Karus Therapeutics Limited | Polyheteroarl histone deacetylase inhibitors and their use in therapy |
| US10596161B2 (en) | 2017-12-08 | 2020-03-24 | Incyte Corporation | Low dose combination therapy for treatment of myeloproliferative neoplasms |
| US10668077B2 (en) | 2015-08-19 | 2020-06-02 | Karus Therapeutics Limited | Compositions comprising phosphoinositide 3-kinase inhibitors and a second antiproliferative agent |
| US10709711B2 (en) | 2013-03-15 | 2020-07-14 | G1 Therapeutics, Inc. | Highly active anti-neoplastic and anti-proliferative agents |
| RU2730849C1 (en) * | 2017-03-23 | 2020-08-26 | Даегу-Гиеонгбук Медикал Инновейшн Фаундейшн | Pyrrolo-pyridine compound derivative, method for production thereof and pharmaceutical composition containing same as active ingredient for preventing and treating protein kinase related diseases |
| US10758543B2 (en) | 2010-05-21 | 2020-09-01 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| WO2021011448A1 (en) * | 2019-07-12 | 2021-01-21 | The Johns Hopkins University | Compositions and methods for treating cerebral vasospasm |
| US10899736B2 (en) | 2018-01-30 | 2021-01-26 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US10988479B1 (en) | 2020-06-15 | 2021-04-27 | G1 Therapeutics, Inc. | Morphic forms of trilaciclib and methods of manufacture thereof |
| US11304949B2 (en) | 2018-03-30 | 2022-04-19 | Incyte Corporation | Treatment of hidradenitis suppurativa using JAK inhibitors |
| US11390609B2 (en) | 2017-06-30 | 2022-07-19 | Beijing Tide Pharmaceutical Co., Ltd. | Rho-associated protein kinase inhibitor, pharmaceutical composition comprising same, and preparation method and use thereof |
| WO2023145372A1 (en) | 2022-01-28 | 2023-08-03 | 日本曹達株式会社 | Method for producing amide compound |
| US11738026B2 (en) | 2019-11-22 | 2023-08-29 | Incyte Corporation | Combination therapy comprising an ALK2 inhibitor and a JAK2 inhibitor |
| US11779586B2 (en) | 2015-08-19 | 2023-10-10 | Convalife (Shanghai) Co. Limited | Compounds comprising tricyclic heterocyclic compounds |
| US11833155B2 (en) | 2020-06-03 | 2023-12-05 | Incyte Corporation | Combination therapy for treatment of myeloproliferative neoplasms |
| US12194048B2 (en) | 2020-12-09 | 2025-01-14 | Voronoi Co., Ltd. | Use of pyrrolopyridine derivatives for preventing or treating inflammatory diseases |
| US12240844B2 (en) | 2019-01-18 | 2025-03-04 | Voronoi, Inc. | Pyrrolopyridine derivative and use thereof in prevention and treatment of protein kinase-related disease |
| US12275731B2 (en) | 2018-10-16 | 2025-04-15 | Dana-Farber Cancer Institute, Inc. | Azaindole inhibitors of wild-type and mutant forms of LRRK2 |
Families Citing this family (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006127587A1 (en) * | 2005-05-20 | 2006-11-30 | Vertex Pharmaceuticals Incorporated | Pyrrolopyridines useful as inhibitors of protein kinase |
| EP2001884A1 (en) * | 2006-04-05 | 2008-12-17 | Vertex Pharmaceuticals, Inc. | Deazapurines useful as inhibitors of janus kinases |
| CA2656566C (en) | 2006-07-06 | 2014-06-17 | Array Biopharma Inc. | Dihydrofuro pyrimidines as akt protein kinase inhibitors |
| US8063050B2 (en) | 2006-07-06 | 2011-11-22 | Array Biopharma Inc. | Hydroxylated and methoxylated pyrimidyl cyclopentanes as AKT protein kinase inhibitors |
| NZ573979A (en) * | 2006-07-06 | 2012-02-24 | Array Biopharma Inc | Cyclopenta [d] pyrimidines as akt protein kinase inhibitors |
| SI2054418T1 (en) | 2006-07-06 | 2012-02-29 | Array Biopharma Inc | Dihydrothieno pyrimidines as akt protein kinase inhibitors |
| US9409886B2 (en) | 2007-07-05 | 2016-08-09 | Array Biopharma Inc. | Pyrimidyl cyclopentanes as AKT protein kinase inhibitors |
| EP2404907B1 (en) * | 2007-07-05 | 2015-01-14 | Array Biopharma, Inc. | Pyrimidyl cyclopentanes as AKT protein kinase inhibitors |
| US8846683B2 (en) | 2007-07-05 | 2014-09-30 | Array Biopharma, Inc. | Pyrimidyl cyclopentanes as Akt protein kinase inhibitors |
| CN101801955B (en) | 2007-07-05 | 2013-05-08 | 阵列生物制药公司 | Pyrimidyl cyclopentanes as akt protein kinase inhibitors |
| EP2242755B1 (en) | 2008-01-08 | 2012-09-12 | Array Biopharma, Inc. | Pyrrolopyridines as kinase inhibitors |
| WO2009089459A1 (en) * | 2008-01-09 | 2009-07-16 | Array Biopharma Inc. | Hydroxylated pyrimidyl cyclopentanes as akt protein kinase inhibitors |
| ES2392014T3 (en) * | 2008-01-09 | 2012-12-03 | Array Biopharma, Inc. | Pyrazolopyridines as kinase inhibitors |
| KR101624752B1 (en) * | 2008-01-09 | 2016-05-26 | 어레이 바이오파마 인크. | Hydroxylated pyrimidyl cyclopentane as akt protein kinase inhibitor |
| GB0801416D0 (en) * | 2008-01-25 | 2008-03-05 | Piramed Ltd | Pharmaceutical compounds |
| EP2262498A2 (en) * | 2008-03-10 | 2010-12-22 | Vertex Pharmceuticals Incorporated | Pyrimidines and pyridines useful as inhibitors of protein kinases |
| CL2009001884A1 (en) * | 2008-10-02 | 2010-05-14 | Incyte Holdings Corp | Use of 3-cyclopentyl-3- [4- (7h-pyrrolo [2,3-d] pyrimidin-4-yl) -1h-pyrazol-1-yl) propanonitrile, janus kinase inhibitor, and use of a composition that understands it for the treatment of dry eye. |
| KR101481872B1 (en) | 2009-09-10 | 2015-01-12 | 에프. 호프만-라 로슈 아게 | Inhibitors of jak |
| USRE49251E1 (en) | 2010-01-04 | 2022-10-18 | Mapi Pharma Ltd. | Depot systems comprising glatiramer or pharmacologically acceptable salt thereof |
| EA023444B1 (en) * | 2010-02-18 | 2016-06-30 | Инсайт Холдингс Корпорейшн | Cyclobutane and methylcyclobutane derivatives, composition based thereon and methods of use thereof |
| EP2397482A1 (en) * | 2010-06-15 | 2011-12-21 | Almirall, S.A. | Heteroaryl imidazolone derivatives as jak inhibitors |
| CN110433165A (en) | 2011-04-01 | 2019-11-12 | 基因泰克公司 | The combination and its application method of AKT and mek inhibitor compound |
| PT2694072T (en) | 2011-04-01 | 2018-02-26 | Genentech Inc | Combination of akt inhibitor compound and abiraterone for use in therapeutic treatments |
| WO2014013014A1 (en) | 2012-07-18 | 2014-01-23 | Fundació Privada Centre De Regulació Genòmica (Crg) | Jak inhibitors for activation of epidermal stem cell populations |
| CN105517549B (en) * | 2013-03-06 | 2018-11-23 | 阿略斯泰罗斯医疗公司 | CaMKII inhibitor and its purposes |
| US9617260B2 (en) * | 2013-04-02 | 2017-04-11 | Hoffmann-La Roche Inc. | Inhibitors of bruton's tyrosine kinase |
| WO2014178426A1 (en) * | 2013-05-02 | 2014-11-06 | 富士フイルム株式会社 | Etching method, etching liquid and etching liquid kit to be used in said method, and semiconductor substrate product manufacturing method |
| RU2674027C2 (en) * | 2014-05-28 | 2018-12-04 | Астразенека Аб | Method of obtaining azd5363 (options) and new intermediate compound applicable therein |
| CN107074856A (en) | 2014-09-05 | 2017-08-18 | 阿略斯泰罗斯医疗公司 | CaMKII inhibitor and application thereof |
| CN105837572B (en) * | 2015-02-02 | 2019-04-19 | 四川大学 | N-substituted phenylamide derivatives and their preparation and use |
| JP6860267B2 (en) | 2015-12-11 | 2021-04-14 | シチュアン ケルン−バイオテック バイオファーマシューティカル カンパニー リミテッド | Azetidine derivatives, their preparation methods, and their use |
| WO2017114275A1 (en) * | 2015-12-31 | 2017-07-06 | 成都先导药物开发有限公司 | Compound for inhibiting rock, preparation method and use of same |
| KR102492378B1 (en) * | 2016-07-26 | 2023-01-27 | 톈진 롱보진 파마시우티컬 씨오., 엘티디. | Compounds as Selective JAK Inhibitors, and Salts and Therapeutic Uses thereof |
| US12097292B2 (en) | 2016-08-28 | 2024-09-24 | Mapi Pharma Ltd. | Process for preparing microparticles containing glatiramer acetate |
| WO2018041989A1 (en) | 2016-09-02 | 2018-03-08 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for diagnosing and treating refractory celiac disease type 2 |
| EP3600553A4 (en) | 2017-03-26 | 2020-09-02 | Mapi Pharma Ltd. | GLATIRAMER DEPOT SYSTEMS FOR TREATMENT OF PROGRESSIVE FORMS OF MULTIPLE SCLEROSIS |
| HUE057818T2 (en) * | 2017-08-23 | 2022-06-28 | Sprint Bioscience Ab | Azaindolylpyridone and diazaindolylpyridone compounds |
| US10800775B2 (en) * | 2017-11-03 | 2020-10-13 | Aclaris Therapeutics, Inc. | Pyrazolyl pyrrolo[2,3-b]pyrmidine-5-carboxylate analogs and methods of making the same |
| KR102821964B1 (en) | 2017-11-03 | 2025-06-19 | 어클라리스 쎄라퓨틱스, 인코포레이티드 | Substituted pyrrolopyrimidine JAK inhibitors and methods for their manufacture and use |
| BR112021002479A2 (en) | 2018-08-10 | 2021-07-27 | Aclaris Therapeutics, Inc. | compound, pharmaceutical composition, method of inhibiting the activity of itk or jak3 in a population of cells, method of treating an itk or jak3 mediated disorder in an individual in need thereof, and use of a compound |
| JP2022527972A (en) | 2019-04-02 | 2022-06-07 | アンスティチュ ナショナル ドゥ ラ サンテ エ ドゥ ラ ルシェルシュ メディカル | How to predict and prevent cancer in patients with premalignant lesions |
| US20220202820A1 (en) | 2019-04-16 | 2022-06-30 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of jak inhibitors for the treatment of painful conditions involving nav1.7 channels |
| WO2020223728A1 (en) * | 2019-05-02 | 2020-11-05 | Aclaris Therapeutics, Inc. | Substituted pyrrolopyridines as jak inhibitors |
| EP4149548A4 (en) | 2020-05-13 | 2024-05-08 | Disc Medicine, Inc. | ANTI-HEMOJUVELIN (HJV) ANTIBODIES FOR THE TREATMENT OF MYELOFIBROSIS |
| CN113402523B (en) * | 2021-07-13 | 2022-07-12 | 西安交通大学 | A small molecule fluorescent probe targeting mast cell MrgX2 and preparation method and application |
| CN113735859A (en) * | 2021-08-12 | 2021-12-03 | 安徽医科大学 | Kinase inhibitor |
| WO2023222565A1 (en) | 2022-05-16 | 2023-11-23 | Institut National de la Santé et de la Recherche Médicale | Methods for assessing the exhaustion of hematopoietic stems cells induced by chronic inflammation |
Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5304121A (en) | 1990-12-28 | 1994-04-19 | Boston Scientific Corporation | Drug delivery system making use of a hydrogel polymer coating |
| US5886026A (en) | 1993-07-19 | 1999-03-23 | Angiotech Pharmaceuticals Inc. | Anti-angiogenic compositions and methods of use |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| WO2001002369A2 (en) * | 1999-07-02 | 2001-01-11 | Agouron Pharmaceuticals, Inc. | Indazole compounds and pharmaceutical compositions for inhibiting protein kinases, and methods for their use |
| WO2001081345A1 (en) * | 2000-04-20 | 2001-11-01 | Mitsubishi Pharma Corporation | Aromatic amide compounds |
| WO2003000688A1 (en) * | 2001-06-21 | 2003-01-03 | Aventis Pharma Limited | Azaindoles |
| WO2004099205A1 (en) * | 2003-05-09 | 2004-11-18 | Astrazeneca Ab | Azaindole compounds as kinase inhibitors |
| WO2005013986A1 (en) * | 2003-08-08 | 2005-02-17 | Pharmacia Italia S.P.A. | Pyridylpyrrole derivatives active as kinase inhibitors |
| WO2005051304A2 (en) * | 2003-11-21 | 2005-06-09 | Array Biopharma Inc. | Akt protein kinase inhibitors |
| WO2005062795A2 (en) * | 2003-12-19 | 2005-07-14 | Plexxikon, Inc. | Compounds and methods for development of ret modulators |
| WO2005108397A1 (en) * | 2004-04-27 | 2005-11-17 | Bayer Healthcare Ag | Substituted phenylamino-pyrimidines |
| WO2005113494A2 (en) * | 2004-05-07 | 2005-12-01 | Amgen Inc. | Nitrogenated heterocyclic derivatives as protein kinase modulators and use for the treatment of angiogenesis and cancer |
| WO2006026754A2 (en) * | 2004-09-03 | 2006-03-09 | Plexxikon, Inc. | Bicyclic heteroaryl pde4b inhibitors |
| US20060084650A1 (en) * | 2004-10-15 | 2006-04-20 | Qing Dong | Kinase inhibitors |
| WO2006046024A1 (en) * | 2004-10-25 | 2006-05-04 | Astex Therapeutics Limited | Ortho- condensed pyridine and pyrimidine derivatives (e. g. purines) as protein kinases inhibitors |
| WO2006046023A1 (en) * | 2004-10-25 | 2006-05-04 | Astex Therapeutics Limited | Ortho- condensed pyridine and pyrimidine derivatives (e. g. purines) as protein kinases inhibitors |
| WO2006052568A2 (en) * | 2004-11-10 | 2006-05-18 | Eli Lilly And Company | Tgf-beta inhibitors |
| WO2006069080A2 (en) * | 2004-12-22 | 2006-06-29 | Incyte Corporation | Pyrrolo[2,3-b]pyridin-4-yl-amines and pyrrolo[2m3-b]pyrimidin-4-yl-amines as janus kinase inhibitors |
| WO2006071548A2 (en) * | 2004-12-27 | 2006-07-06 | Alcon, Inc. | Aminopyrazine analogs for treating glaucoma and other rho kinase-mediated diseases |
Family Cites Families (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SK287882B6 (en) | 1999-12-24 | 2012-02-03 | Aventis Pharma Limited | Azaindoles derivatives, pharmaceutical composition containing thereof and their use |
| US6335342B1 (en) * | 2000-06-19 | 2002-01-01 | Pharmacia & Upjohn S.P.A. | Azaindole derivatives, process for their preparation, and their use as antitumor agents |
| AU9598601A (en) | 2000-10-20 | 2002-04-29 | Eisai Co Ltd | Nitrogenous aromatic ring compounds |
| GB0202679D0 (en) | 2002-02-05 | 2002-03-20 | Glaxo Group Ltd | Novel compounds |
| AU2003237121A1 (en) * | 2002-04-26 | 2003-11-10 | Vertex Pharmaceuticals Incorporated | Pyrrole derivatives as inhibitors of erk2 and uses thereof |
| AU2003278088A1 (en) | 2002-10-28 | 2004-05-25 | Bayer Healthcare Ag | Heteroaryloxy-substituted phenylaminopyrimidines as rho-kinase inhibitors |
| GB0227240D0 (en) | 2002-11-21 | 2002-12-31 | Glaxo Group Ltd | Compounds |
| EP1581526B1 (en) * | 2002-12-18 | 2009-03-11 | Vertex Pharmaceuticals Incorporated | Benzisoxazole derivatives useful as inhibitors of protein kinases |
| AU2004212421B2 (en) * | 2003-02-07 | 2009-08-20 | Vertex Pharmaceuticals Incorporated | Heteroaryl substituted pyrolls useful as inhibitors of protein kinases |
| BRPI0407493A (en) * | 2003-02-14 | 2006-02-14 | Wyeth Corp | heterocyclyl-3-sulfinylazaindole or -azaindazole derivatives as 5-hydroxytryptamine-6 binders |
| US7531553B2 (en) * | 2003-03-21 | 2009-05-12 | Amgen Inc. | Heterocyclic compounds and methods of use |
| FR2857661B1 (en) * | 2003-07-15 | 2006-09-22 | Snc Eurokera | PRODUCT IN VITROCERAMIC WITH SEAL (S); MANUFACTURING |
| TWI339206B (en) * | 2003-09-04 | 2011-03-21 | Vertex Pharma | Compositions useful as inhibitors of protein kinases |
| FR2861729B1 (en) * | 2003-10-31 | 2006-09-08 | Fabre Pierre Dermo Cosmetique | ALKYL-RHAMNOSE MONOMER OR ALKYL-FUCOSE, MEDICAMENT COMPRISING AN ALKYL-SUGAR MONOMER REDUCING |
| FR2862363B1 (en) * | 2003-11-18 | 2007-01-26 | Antonov Automotive Europ | MULTI-REPORTING TRANSMISSION DEVICE, IN PARTICULAR FOR THE AUTOMOBILE |
| FR2868273B1 (en) * | 2004-03-30 | 2006-07-07 | Parfums Christian Dior Sa | DISPLAY OF ARTICLES SUCH AS COSMETIC PRODUCTS |
| PT2332940E (en) | 2004-03-30 | 2013-01-30 | Vertex Pharma | Azaindoles useful as inhibitors of jak and other protein kinases |
| AU2005236002A1 (en) * | 2004-04-02 | 2005-11-03 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of rock and other protein kinases |
| US20060122213A1 (en) * | 2004-06-30 | 2006-06-08 | Francoise Pierard | Azaindoles useful as inhibitors of protein kinases |
| TW200615268A (en) | 2004-08-02 | 2006-05-16 | Osi Pharm Inc | Aryl-amino substituted pyrrolopyrimidine multi-kinase inhibiting compounds |
| FR2874920B1 (en) * | 2004-09-09 | 2006-10-20 | Sanofi Aventis Sa | 3-SPIRO-INDOLIN-2-ONE DERIVATIVES AS LIGAND OF VASOPRESSIN RECEPTORS |
| DE602005022472D1 (en) | 2004-09-22 | 2010-09-02 | Janssen Pharmaceutica Nv | INHIBITORS OF INTERACTION BETWEEN MDM2 AND P53 |
| SI1812440T1 (en) * | 2004-11-04 | 2011-04-29 | Vertex Pharma | Pyrazolo[1,5-a] pyrimidines useful as inhibitors of protein kinases |
| AU2005309566A1 (en) * | 2004-11-22 | 2006-06-01 | Vertex Pharmaceuticals Incorporated | Bicyclic inhibitors or Rho kinase |
| GB0501999D0 (en) * | 2005-02-01 | 2005-03-09 | Sentinel Oncology Ltd | Pharmaceutical compounds |
| KR20070104641A (en) * | 2005-02-03 | 2007-10-26 | 버텍스 파마슈티칼스 인코포레이티드 | Pyrrolopyrimidines useful as inhibitors of protein kinases |
| DE602006014540D1 (en) * | 2005-05-16 | 2010-07-08 | Irm Llc | Pyrrolopyridinderivate als proteinkinaseinhibitoren |
| WO2006127587A1 (en) * | 2005-05-20 | 2006-11-30 | Vertex Pharmaceuticals Incorporated | Pyrrolopyridines useful as inhibitors of protein kinase |
| AU2006297351A1 (en) * | 2005-09-30 | 2007-04-12 | Vertex Pharmaceuticals Incorporated | Deazapurines useful as inhibitors of janus kinases |
| TWI405761B (en) * | 2006-01-17 | 2013-08-21 | Vertex Pharma | Azaindoles useful as inhibitors of janus kinases |
| TW200738709A (en) * | 2006-01-19 | 2007-10-16 | Osi Pharm Inc | Fused heterobicyclic kinase inhibitors |
| EP2001884A1 (en) * | 2006-04-05 | 2008-12-17 | Vertex Pharmaceuticals, Inc. | Deazapurines useful as inhibitors of janus kinases |
| AR064675A1 (en) * | 2006-12-21 | 2009-04-15 | Vertex Pharma | PIRROLO HETEROCICLIC DERIVATIVES- [2,3-B] PIRIDINE, PLK PROTEINQUINASE INHIBITORS, PHARMACEUTICAL COMPOSITIONS CONTAINING THEM, PREPARATION PROCESS AND USE OF THEM IN HYPERPROLIFERATIVE DISORDERS |
| JP2009123635A (en) * | 2007-11-19 | 2009-06-04 | Nissan Diesel Motor Co Ltd | Harness assembly |
| KR101903354B1 (en) * | 2009-06-17 | 2018-10-04 | 버텍스 파마슈티칼스 인코포레이티드 | Inhibitors of influenza viruses replication |
| CN103562205A (en) * | 2010-12-16 | 2014-02-05 | 沃泰克斯药物股份有限公司 | Inhibitors of influenza viruses replication |
| UA118010C2 (en) * | 2011-08-01 | 2018-11-12 | Вертекс Фармасьютікалз Інкорпорейтед | INFLUENCES OF INFLUENZA VIRUS REPLICATION |
-
2006
- 2006-05-22 WO PCT/US2006/019711 patent/WO2006127587A1/en active Application Filing
- 2006-05-22 JP JP2008512591A patent/JP2008545660A/en active Pending
- 2006-05-22 CN CN2006800263918A patent/CN101228161B/en not_active Expired - Fee Related
- 2006-05-22 CA CA002609126A patent/CA2609126A1/en not_active Abandoned
- 2006-05-22 EP EP10188694A patent/EP2354139A1/en not_active Withdrawn
- 2006-05-22 EP EP06770824A patent/EP1881983B1/en not_active Not-in-force
- 2006-05-22 ZA ZA200710379A patent/ZA200710379B/en unknown
- 2006-05-22 AU AU2006251623A patent/AU2006251623A1/en not_active Abandoned
- 2006-05-22 NZ NZ564065A patent/NZ564065A/en not_active IP Right Cessation
- 2006-05-22 AT AT06770824T patent/ATE540948T1/en active
- 2006-05-22 RU RU2007147455/04A patent/RU2435769C2/en not_active IP Right Cessation
- 2006-05-22 US US11/438,748 patent/US8921376B2/en not_active Expired - Fee Related
- 2006-05-22 MX MX2007014619A patent/MX2007014619A/en not_active Application Discontinuation
- 2006-05-22 EP EP10188700A patent/EP2354140A1/en not_active Withdrawn
- 2006-05-22 ES ES06770824T patent/ES2380795T3/en active Active
- 2006-05-22 KR KR1020077029764A patent/KR20080016659A/en not_active Ceased
-
2007
- 2007-11-18 IL IL187438A patent/IL187438A0/en unknown
- 2007-12-18 NO NO20076502A patent/NO20076502L/en not_active Application Discontinuation
-
2009
- 2009-05-21 JP JP2009123635A patent/JP2009179644A/en active Pending
-
2011
- 2011-08-11 RU RU2011133825/04A patent/RU2011133825A/en not_active Application Discontinuation
-
2013
- 2013-09-24 JP JP2013196681A patent/JP2013253114A/en active Pending
Patent Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5304121A (en) | 1990-12-28 | 1994-04-19 | Boston Scientific Corporation | Drug delivery system making use of a hydrogel polymer coating |
| US5886026A (en) | 1993-07-19 | 1999-03-23 | Angiotech Pharmaceuticals Inc. | Anti-angiogenic compositions and methods of use |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| WO2001002369A2 (en) * | 1999-07-02 | 2001-01-11 | Agouron Pharmaceuticals, Inc. | Indazole compounds and pharmaceutical compositions for inhibiting protein kinases, and methods for their use |
| WO2001081345A1 (en) * | 2000-04-20 | 2001-11-01 | Mitsubishi Pharma Corporation | Aromatic amide compounds |
| WO2003000688A1 (en) * | 2001-06-21 | 2003-01-03 | Aventis Pharma Limited | Azaindoles |
| WO2004099205A1 (en) * | 2003-05-09 | 2004-11-18 | Astrazeneca Ab | Azaindole compounds as kinase inhibitors |
| WO2005013986A1 (en) * | 2003-08-08 | 2005-02-17 | Pharmacia Italia S.P.A. | Pyridylpyrrole derivatives active as kinase inhibitors |
| WO2005051304A2 (en) * | 2003-11-21 | 2005-06-09 | Array Biopharma Inc. | Akt protein kinase inhibitors |
| WO2005062795A2 (en) * | 2003-12-19 | 2005-07-14 | Plexxikon, Inc. | Compounds and methods for development of ret modulators |
| WO2005108397A1 (en) * | 2004-04-27 | 2005-11-17 | Bayer Healthcare Ag | Substituted phenylamino-pyrimidines |
| WO2005113494A2 (en) * | 2004-05-07 | 2005-12-01 | Amgen Inc. | Nitrogenated heterocyclic derivatives as protein kinase modulators and use for the treatment of angiogenesis and cancer |
| WO2006026754A2 (en) * | 2004-09-03 | 2006-03-09 | Plexxikon, Inc. | Bicyclic heteroaryl pde4b inhibitors |
| US20060084650A1 (en) * | 2004-10-15 | 2006-04-20 | Qing Dong | Kinase inhibitors |
| WO2006046024A1 (en) * | 2004-10-25 | 2006-05-04 | Astex Therapeutics Limited | Ortho- condensed pyridine and pyrimidine derivatives (e. g. purines) as protein kinases inhibitors |
| WO2006046023A1 (en) * | 2004-10-25 | 2006-05-04 | Astex Therapeutics Limited | Ortho- condensed pyridine and pyrimidine derivatives (e. g. purines) as protein kinases inhibitors |
| WO2006052568A2 (en) * | 2004-11-10 | 2006-05-18 | Eli Lilly And Company | Tgf-beta inhibitors |
| WO2006069080A2 (en) * | 2004-12-22 | 2006-06-29 | Incyte Corporation | Pyrrolo[2,3-b]pyridin-4-yl-amines and pyrrolo[2m3-b]pyrimidin-4-yl-amines as janus kinase inhibitors |
| WO2006071548A2 (en) * | 2004-12-27 | 2006-07-06 | Alcon, Inc. | Aminopyrazine analogs for treating glaucoma and other rho kinase-mediated diseases |
Non-Patent Citations (1)
| Title |
|---|
| THUTEWOHL, MICHAEL; SCHIROK, HARTMUT; BENNABI, SAMIR; FIGUEROA-PEREZ, SANTIAGO: "Synthesis of 4-substituted 7-azaindole derivatives via Pd-catalyzed C-N and C-O coupling", SYNTHESIS, vol. 4, 2006, pages 629 - 632, XP002399436 * |
Cited By (307)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7504509B2 (en) | 2003-12-19 | 2009-03-17 | Plexxikon, Inc. | Compounds and methods for development of Ret modulators |
| US8067434B2 (en) | 2003-12-19 | 2011-11-29 | Plexxikon Inc. | Compounds and methods for development of Ret modulators |
| US8987454B2 (en) | 2004-03-30 | 2015-03-24 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of JAK and other protein kinases |
| US8722889B2 (en) | 2004-03-30 | 2014-05-13 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of JAK and other protein kinases |
| US8188281B2 (en) | 2004-03-30 | 2012-05-29 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of JAK and other protein kinases |
| US8501446B2 (en) | 2004-03-30 | 2013-08-06 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of JAK and other protein kinases |
| US7947708B2 (en) | 2004-06-17 | 2011-05-24 | Plexxikon, Inc. | Compounds modulating C-kit activity |
| US7498342B2 (en) | 2004-06-17 | 2009-03-03 | Plexxikon, Inc. | Compounds modulating c-kit activity |
| US7846941B2 (en) | 2005-05-17 | 2010-12-07 | Plexxikon, Inc. | Compounds modulating c-kit and c-fms activity and uses therefor |
| US8470818B2 (en) | 2005-06-22 | 2013-06-25 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US8415469B2 (en) | 2005-06-22 | 2013-04-09 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US7863288B2 (en) | 2005-06-22 | 2011-01-04 | Plexxikon, Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US8163767B2 (en) | 2005-07-14 | 2012-04-24 | Astellas Pharma Inc. | Heterocyclic Janus Kinase 3 inhibitors |
| EP1910358A2 (en) * | 2005-07-14 | 2008-04-16 | Astellas Pharma Inc. | Heterocyclic janus kinase 3 inhibitors |
| EP2251341A1 (en) * | 2005-07-14 | 2010-11-17 | Astellas Pharma Inc. | Heterocyclic Janus kinase 3 inhibitors |
| US8563541B2 (en) | 2005-09-22 | 2013-10-22 | Incyte Corporation | Azepine inhibitors of Janus kinases |
| US8835423B2 (en) | 2005-09-22 | 2014-09-16 | Incyte Corporation | Azepine inhibitors of janus kinases |
| US7598257B2 (en) | 2005-12-13 | 2009-10-06 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors |
| JP2011252024A (en) * | 2005-12-13 | 2011-12-15 | Incyte Corp | HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINE AND PYRROLO[2,3-b]PYRIMIDINE AS JANUS KINASE INHIBITORS |
| EP3184526A1 (en) * | 2005-12-13 | 2017-06-28 | Incyte Holdings Corporation | Pyrrolo[2,3-d]pyrimidine derivatives as janus kinase inhibitor |
| JP2015193641A (en) * | 2005-12-13 | 2015-11-05 | インサイト・コーポレイションIncyte Corpor | HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINE AND PYRROLO[2,3-b]PYRIMIDINE AS JANUS KINASE INHIBITORS |
| US8933086B2 (en) | 2005-12-13 | 2015-01-13 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-B]pyridines and pyrrolo[2,3-B]pyrimidines as Janus kinase inhibitors |
| US10398699B2 (en) | 2005-12-13 | 2019-09-03 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors |
| US11331320B2 (en) | 2005-12-13 | 2022-05-17 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| US9206187B2 (en) | 2005-12-13 | 2015-12-08 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as Janus kinase |
| US8946245B2 (en) | 2005-12-13 | 2015-02-03 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| US9662335B2 (en) | 2005-12-13 | 2017-05-30 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as janus kinase inhibitors |
| JP2014051531A (en) * | 2005-12-13 | 2014-03-20 | Incyte Corp | HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINE AND PYRROLO[2,3-b]PYRIMIDINE AS JANUS KINASE INHIBITORS |
| US10639310B2 (en) | 2005-12-13 | 2020-05-05 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| US9974790B2 (en) | 2005-12-13 | 2018-05-22 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as janus kinase inhibitors |
| WO2007070514A1 (en) * | 2005-12-13 | 2007-06-21 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors |
| EP3466953A1 (en) * | 2005-12-13 | 2019-04-10 | Incyte Holdings Corporation | Pyrrolo[2,3-d]pyrimidine derivative as janus kinase inhibitor |
| EP3838903A1 (en) * | 2005-12-13 | 2021-06-23 | Incyte Holdings Corporation | Pyrrolo[2,3-d]pyrimidine derivative as janus kinase inhibitor |
| EA019504B1 (en) * | 2005-12-13 | 2014-04-30 | Инсайт Корпорейшн | HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS |
| US11744832B2 (en) | 2005-12-13 | 2023-09-05 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| EP2474545A1 (en) * | 2005-12-13 | 2012-07-11 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| JP2009519340A (en) * | 2005-12-13 | 2009-05-14 | インサイト・コーポレイション | Heteroaryl-substituted pyrrolo [2,3-b] pyridines and pyrrolo [2,3-b] pyrimidines as JANUS kinase inhibitors |
| US9814722B2 (en) | 2005-12-13 | 2017-11-14 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as janus kinase inhibitors |
| EP2343298A1 (en) * | 2005-12-13 | 2011-07-13 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| EP2343299A1 (en) * | 2005-12-13 | 2011-07-13 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| EP2348023A1 (en) * | 2005-12-13 | 2011-07-27 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| EP2455382A1 (en) * | 2005-12-13 | 2012-05-23 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| US9079912B2 (en) | 2005-12-13 | 2015-07-14 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as Janus kinase inhibitors |
| AU2006326548B2 (en) * | 2005-12-13 | 2012-04-05 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| EP2426129A1 (en) * | 2005-12-13 | 2012-03-07 | Incyte Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| AU2015201850B2 (en) * | 2005-12-13 | 2017-03-02 | Incyte Holdings Corporation | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors |
| EP1968568A4 (en) * | 2005-12-22 | 2011-04-13 | Glaxosmithkline Llc | INHIBITORS OF Akt ACTIVITY |
| WO2007077949A1 (en) * | 2005-12-28 | 2007-07-12 | Astellas Pharma Inc. | Heterocyclic janus kinase 3 inhibitors |
| JP2009522206A (en) * | 2005-12-28 | 2009-06-11 | アステラス製薬株式会社 | Heterocyclic Janus kinase 3 inhibitor |
| US7879844B2 (en) | 2005-12-28 | 2011-02-01 | Astellas Pharma Inc. | Heterocyclic janus kinase 3 inhibitors |
| NO341555B1 (en) * | 2005-12-28 | 2017-12-04 | Astellas Pharma Inc | Heterocyclic januskinase-3 inhibitors |
| AU2006334044B2 (en) * | 2005-12-28 | 2012-02-02 | Astellas Pharma Inc. | Heterocyclic janus kinase 3 inhibitors |
| US9120790B2 (en) | 2006-01-17 | 2015-09-01 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of Janus kinases |
| US7767816B2 (en) | 2006-01-17 | 2010-08-03 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of janus kinases |
| US8450489B2 (en) | 2006-01-17 | 2013-05-28 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of janus kinases |
| US8822681B2 (en) | 2006-01-17 | 2014-09-02 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of janus kinases |
| WO2007084557A3 (en) * | 2006-01-17 | 2007-09-27 | Vertex Pharma | Azaindoles useful as inhibitors of janus kinases |
| US8163917B2 (en) | 2006-01-17 | 2012-04-24 | Vertex Pharmaceuticals Incorporated | Azaindoles useful as inhibitors of Janus kinases |
| JP2009523753A (en) * | 2006-01-18 | 2009-06-25 | エフ.ホフマン−ラ ロシュ アーゲー | Thiazole as an 11 beta-HSD1 inhibitor |
| AU2007207055B2 (en) * | 2006-01-18 | 2011-06-02 | F. Hoffmann-La Roche Ag | Thiazoles as 11 beta-HSD1 inhibitors |
| US7645773B2 (en) | 2006-01-18 | 2010-01-12 | Hoffmann-La Roche Inc. | Thiazoles as inhibitors of 11β-hydroxysteroid dehydrogenase |
| WO2007082808A3 (en) * | 2006-01-18 | 2007-09-13 | Hoffmann La Roche | Thiazoles as 11 beta-hsd1 inhibitors |
| JP2009523812A (en) * | 2006-01-19 | 2009-06-25 | オーエスアイ・ファーマスーティカルズ・インコーポレーテッド | Fusion heterobicyclic kinase inhibitors |
| WO2007084667A3 (en) * | 2006-01-19 | 2007-12-06 | Osi Pharmaceutical Inc | Fused heterobicyclic kinase inhibitors |
| WO2007136790A3 (en) * | 2006-05-18 | 2008-11-27 | Mannkind Corp | Intracellular kinase inhibitors |
| JP2010509356A (en) * | 2006-11-10 | 2010-03-25 | ブリストル−マイヤーズ スクイブ カンパニー | Novel kinase inhibitor |
| US8722702B2 (en) | 2006-11-22 | 2014-05-13 | Plexxikon Inc. | Compounds modulating c-fms and/or c-kit activity and uses therefor |
| US8404700B2 (en) | 2006-11-22 | 2013-03-26 | Plexxikon Inc. | Compounds modulating c-fms and/or c-kit activity and uses therefor |
| US9487515B2 (en) | 2006-11-22 | 2016-11-08 | Plexxikon Inc. | Compounds modulating c-fms and/or c-kit activity and uses therefor |
| US8461169B2 (en) | 2006-11-22 | 2013-06-11 | Plexxikon Inc. | Compounds modulating c-fms and/or c-kit activity |
| US7893075B2 (en) | 2006-11-22 | 2011-02-22 | Plexxikon, Inc. | Compounds modulating c-fms and/or c-kit activity and uses therefor |
| US9169250B2 (en) | 2006-11-22 | 2015-10-27 | Plexxikon Inc. | Compounds modulating c-fms and/or c-kit activity and uses therefor |
| JP2010513319A (en) * | 2006-12-18 | 2010-04-30 | インスパイアー ファーマシューティカルズ,インコーポレイティド | Cytoskeletal active RHO kinase inhibitor compounds, compositions and uses |
| US8604218B2 (en) | 2006-12-18 | 2013-12-10 | Inspire Pharmaceuticals, Inc. | Cytoskeletal active rho kinase inhibitor compounds, composition and use |
| US8604205B2 (en) | 2006-12-18 | 2013-12-10 | Inspire Pharmaceuticals, Inc. | Cytoskeletal active rho kinase inhibitor compounds, composition and use |
| US8247421B2 (en) | 2006-12-21 | 2012-08-21 | Vertex Pharmaceuticals Incorporated | 5-cyano-4-(pyrrolo [2,3B] pyridine-3-yl)-pyrimidine derivatives useful as protein kinase inhibitors |
| US8530489B2 (en) | 2006-12-21 | 2013-09-10 | Vertex Pharmaceuticals Incorporated | 5-cyano-4-(pyrrolo [2,3B] pyridine-3-yl)-pyrimidine derivatives useful as protein kinase inhibitors |
| US8268858B2 (en) | 2006-12-21 | 2012-09-18 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US8962642B2 (en) | 2006-12-21 | 2015-02-24 | Vertex Pharmaceuticals Incorporated | 5-cyano-4- (pyrrolo [2,3B] pyridine-3-yl) -pyrimidine derivatives useful as protein kinase inhibitors |
| US7863289B2 (en) | 2006-12-21 | 2011-01-04 | Plexxikon, Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US7872018B2 (en) | 2006-12-21 | 2011-01-18 | Plexxikon, Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US8841318B2 (en) | 2006-12-22 | 2014-09-23 | Incyte Corporation | Substituted heterocycles as janus kinase inhibitors |
| US8513270B2 (en) | 2006-12-22 | 2013-08-20 | Incyte Corporation | Substituted heterocycles as Janus kinase inhibitors |
| WO2008084861A1 (en) | 2007-01-12 | 2008-07-17 | Astellas Pharma Inc. | Condensed pyridine compound |
| WO2008116139A3 (en) * | 2007-03-22 | 2008-12-18 | Vertex Pharma | N-heterocyclic compounds useful as inhibitors of janus kinases |
| US8686143B2 (en) | 2007-03-22 | 2014-04-01 | Vertex Pharmaceuticals Incorporated | Compounds useful as inhibitors of Janus kinases |
| US8653262B2 (en) | 2007-05-31 | 2014-02-18 | Boehringer Ingelheim International Gmbh | CCR2 receptor antagonists and uses thereof |
| WO2008145688A3 (en) * | 2007-06-01 | 2009-03-26 | Glaxo Group Ltd | Pyrrolopyridine compounds, process for their preparation, and their use as medicaments |
| JP2010529002A (en) * | 2007-06-01 | 2010-08-26 | グラクソ グループ リミテッド | Pyrrolopyridine compounds, methods for their preparation, and their use as pharmaceuticals |
| US8722693B2 (en) | 2007-06-13 | 2014-05-13 | Incyte Corporation | Salts of the Janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US9376439B2 (en) | 2007-06-13 | 2016-06-28 | Incyte Corporation | Salts of the janus kinase inhibitor (R)-3(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US11213528B2 (en) | 2007-06-13 | 2022-01-04 | Incyte Holdings Corporation | Salts of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US7834022B2 (en) | 2007-06-13 | 2010-11-16 | Incyte Corporation | Metabolites of the Janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US10463667B2 (en) | 2007-06-13 | 2019-11-05 | Incyte Incorporation | Metabolites of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US10016429B2 (en) | 2007-06-13 | 2018-07-10 | Incyte Corporation | Salts of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US8822481B1 (en) | 2007-06-13 | 2014-09-02 | Incyte Corporation | Salts of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d] pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US8829013B1 (en) | 2007-06-13 | 2014-09-09 | Incyte Corporation | Salts of the Janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US10610530B2 (en) | 2007-06-13 | 2020-04-07 | Incyte Corporation | Salts of the Janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| EP3070090B1 (en) | 2007-06-13 | 2018-12-12 | Incyte Holdings Corporation | Use of salts of the janus kinase inhibitor (r)-3-(4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h- pyrazol-1-yl)-3- cyclopentylpropanenitrile |
| US8889697B2 (en) | 2007-06-13 | 2014-11-18 | Incyte Corporation | Metabolites of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US9844539B2 (en) | 2007-07-17 | 2017-12-19 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US10426760B2 (en) | 2007-07-17 | 2019-10-01 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US9469640B2 (en) | 2007-07-17 | 2016-10-18 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US8158616B2 (en) | 2008-03-11 | 2012-04-17 | Incyte Corporation | Azetidine and cyclobutane derivatives as JAK inhibitors |
| US8420629B2 (en) | 2008-03-11 | 2013-04-16 | Incyte Corporation | Azetidine and cyclobutane derivatives as JAK inhibitors |
| WO2009112473A1 (en) * | 2008-03-12 | 2009-09-17 | Glaxo Group Limited | Novel compounds |
| EP2990407A1 (en) | 2008-05-13 | 2016-03-02 | Array Biopharma, Inc. | Pyrrolopyridines as kinase inhibitors |
| CN104926810A (en) * | 2008-05-13 | 2015-09-23 | 阵列生物制药公司 | Pyrrolopyridines As Kinase Inhibitors |
| US9969727B2 (en) | 2008-05-13 | 2018-05-15 | Array Biopharma Inc. | Pyrrolopyridines as kinase inhibitors |
| US10301285B2 (en) | 2008-06-16 | 2019-05-28 | Gtx, Inc. | Compounds for treatment of cancer |
| US8592465B2 (en) | 2008-06-16 | 2013-11-26 | University Of Tennessee Research Foundation | Compounds for treatment of cancer |
| US9334242B2 (en) | 2008-06-16 | 2016-05-10 | Gtx, Inc. | Compounds for treatment of cancer |
| EP2303021A4 (en) * | 2008-06-16 | 2012-03-14 | Univ Tennessee Res Foundation | COMPOUNDS FOR TREATING CANCER |
| US9029408B2 (en) | 2008-06-16 | 2015-05-12 | Gtx, Inc. | Compounds for treatment of cancer |
| AU2009330686B2 (en) * | 2008-06-16 | 2014-07-03 | The Ohio State University Research Foundation | Compounds for the treatment of cancer |
| US10865196B2 (en) | 2008-06-16 | 2020-12-15 | University Of Tennessee Research Foundation | Compounds for treatment of cancer |
| DE102008052943A1 (en) | 2008-10-23 | 2010-04-29 | Merck Patent Gmbh | azaindole derivatives |
| US8835440B2 (en) | 2008-12-19 | 2014-09-16 | Boehringer Ingelheim International Gmbh | Cyclic pyrimidin-4-carboxamides as CCR2 receptor antagonists for treatment of inflammation, asthma and COPD |
| US9067951B2 (en) | 2008-12-19 | 2015-06-30 | Boehringer Ingelheim International Gmbh | Process and intermediates for the production of CCR2 antagonists |
| US9663517B2 (en) | 2009-04-03 | 2017-05-30 | Plexxikon Inc. | Compositions and uses thereof |
| US9447089B2 (en) | 2009-04-03 | 2016-09-20 | Plexxikon Inc. | Compositions and uses thereof |
| JP2016040271A (en) * | 2009-05-22 | 2016-03-24 | インサイト・コーポレイションIncyte Corpor | 3- [4- (7H-Pyrrolo [2,3-D] pyrimidin-4-yl) -1H-pyrazol-1-yl] octane- or heptane-nitrile as JAK inhibitors |
| US9334274B2 (en) | 2009-05-22 | 2016-05-10 | Incyte Holdings Corporation | N-(hetero)aryl-pyrrolidine derivatives of pyrazol-4-yl-pyrrolo[2,3-d]pyrimidines and pyrrol-3-yl-pyrrolo[2,3-d]pyrimidines as janus kinase inhibitors |
| US9216984B2 (en) | 2009-05-22 | 2015-12-22 | Incyte Corporation | 3-[4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl]octane—or heptane-nitrile as JAK inhibitors |
| US9623029B2 (en) | 2009-05-22 | 2017-04-18 | Incyte Holdings Corporation | 3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]octane- or heptane-nitrile as JAK inhibitors |
| JP2012527481A (en) * | 2009-05-22 | 2012-11-08 | インサイト・コーポレイション | 3- [4- (7H-Pyrrolo [2,3-D] pyrimidin-4-yl) -1H-pyrazol-1-yl] octane- or heptane-nitrile as JAK inhibitors |
| US9518056B2 (en) | 2009-06-17 | 2016-12-13 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US10874673B2 (en) | 2009-06-17 | 2020-12-29 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US8829007B2 (en) | 2009-06-17 | 2014-09-09 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US9345708B2 (en) | 2009-06-17 | 2016-05-24 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US9808459B2 (en) | 2009-06-17 | 2017-11-07 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US10039762B2 (en) | 2009-06-17 | 2018-08-07 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| WO2011003418A1 (en) | 2009-07-08 | 2011-01-13 | Leo Pharma A/S | Heterocyclic compounds as jak receptor and protein tyrosine kinase inhibitors |
| US9346809B2 (en) | 2009-07-08 | 2016-05-24 | Leo Pharma A/S | Heterocyclic compounds as JAK receptor and protein tyrosine kinase inhibitors |
| EP2460806A4 (en) * | 2009-07-31 | 2012-12-26 | Japan Tobacco Inc | SPIRO CYCLE COMPOUND CONTAINING NITROGEN AND ITS MEDICAL USE |
| EP3181570A1 (en) * | 2009-07-31 | 2017-06-21 | Japan Tobacco Inc. | Nitrogen-containing spiro-ring compound and medicinal use of same |
| KR101773208B1 (en) | 2009-07-31 | 2017-08-31 | 니뽄 다바코 산교 가부시키가이샤 | Nitrogen-containing spiro-ring compound and medicinal use of same |
| US8609647B2 (en) | 2009-07-31 | 2013-12-17 | Japan Tobacco Inc. | Nitrogen-containing spirocyclic compounds and pharmaceutical uses thereof |
| CN102510865A (en) * | 2009-07-31 | 2012-06-20 | 日本烟草产业株式会社 | Nitrogen-containing spiro-ring compound and medicinal use of same |
| EP3858349A1 (en) * | 2009-07-31 | 2021-08-04 | Japan Tobacco Inc. | Nitrogen-containing spiro-ring compound and medicinal use of same |
| US8741920B2 (en) | 2009-08-03 | 2014-06-03 | Hoffmann-La Roche, Inc. | Process for the manufacture of pharmaceutically active compounds |
| US8569295B2 (en) | 2009-08-06 | 2013-10-29 | Merck Patent Gmbh | Bicyclic urea compounds |
| WO2011017142A1 (en) * | 2009-08-06 | 2011-02-10 | Merck Patent Gmbh | Novel bicyclic urea compounds |
| CN102573994A (en) * | 2009-08-06 | 2012-07-11 | 默克专利有限公司 | Novel bicyclic urea compounds |
| AU2010281368C1 (en) * | 2009-08-06 | 2016-08-04 | Merck Patent Gmbh | Novel bicyclic urea compounds |
| AU2010281368B2 (en) * | 2009-08-06 | 2016-04-14 | Merck Patent Gmbh | Novel bicyclic urea compounds |
| US9023847B2 (en) | 2009-08-07 | 2015-05-05 | Merck Patent Gmbh | Azaheterocyclic compounds |
| AU2010281503B2 (en) * | 2009-08-07 | 2016-01-28 | Merck Patent Gmbh | Novel azaheterocyclic compounds |
| CN102471338A (en) * | 2009-08-07 | 2012-05-23 | 默克专利有限公司 | Novel azaheterocyclic compounds |
| CN102471338B (en) * | 2009-08-07 | 2014-07-02 | 默克专利有限公司 | Novel azaheterocyclic compounds |
| WO2011017009A1 (en) | 2009-08-07 | 2011-02-10 | Merck Patent Gmbh | Novel azaheterocyclic compounds |
| EA020302B1 (en) * | 2009-08-07 | 2014-10-30 | Мерк Патент Гмбх | Novel azaheterocyclic compounds |
| US9580442B2 (en) | 2009-08-20 | 2017-02-28 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US9938290B2 (en) | 2009-08-20 | 2018-04-10 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US10501478B2 (en) | 2009-08-20 | 2019-12-10 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US9249145B2 (en) | 2009-09-01 | 2016-02-02 | Incyte Holdings Corporation | Heterocyclic derivatives of pyrazol-4-yl-pyrrolo[2,3-d]pyrimidines as janus kinase inhibitors |
| US9512161B2 (en) | 2009-10-09 | 2016-12-06 | Incyte Corporation | Hydroxyl, keto, and glucuronide derivatives of 3-(4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| US9096593B2 (en) | 2009-11-06 | 2015-08-04 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US9670222B2 (en) | 2009-12-17 | 2017-06-06 | Centrexion Therapeutics Corporation | CCR2 receptor antagonists and uses thereof |
| US8822513B2 (en) | 2010-03-01 | 2014-09-02 | Gtx, Inc. | Compounds for treatment of cancer |
| US9447049B2 (en) | 2010-03-01 | 2016-09-20 | University Of Tennessee Research Foundation | Compounds for treatment of cancer |
| US11465987B2 (en) | 2010-03-01 | 2022-10-11 | Oncternal Therapeutics, Inc. | Compounds for treatment of cancer |
| US9464088B2 (en) | 2010-03-10 | 2016-10-11 | Incyte Holdings Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| US11285140B2 (en) | 2010-03-10 | 2022-03-29 | Incyte Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| US10695337B2 (en) | 2010-03-10 | 2020-06-30 | Incyte Holdings Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| US9999619B2 (en) | 2010-03-10 | 2018-06-19 | Incyte Holdings Corporation | Piperidin-4-yl azetidine derivatives as JAK1 inhibitors |
| US8877745B2 (en) | 2010-05-12 | 2014-11-04 | Boehringer Ingelheim International Gmbh | CCR2 receptor antagonists, method for producing the same, and use thereof as medicaments |
| US8946218B2 (en) | 2010-05-12 | 2015-02-03 | Boehringer Ingelheim International Gmbh | CCR2 receptor antagonists, method for producing the same, and use thereof as medicaments |
| US8841313B2 (en) | 2010-05-17 | 2014-09-23 | Boehringer Ingelheim International Gmbh | CCR2 antagonists and uses thereof |
| US11590136B2 (en) | 2010-05-21 | 2023-02-28 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US11571425B2 (en) | 2010-05-21 | 2023-02-07 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US11219624B2 (en) | 2010-05-21 | 2022-01-11 | Incyte Holdings Corporation | Topical formulation for a JAK inhibitor |
| US12226419B2 (en) | 2010-05-21 | 2025-02-18 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US10869870B2 (en) | 2010-05-21 | 2020-12-22 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US10758543B2 (en) | 2010-05-21 | 2020-09-01 | Incyte Corporation | Topical formulation for a JAK inhibitor |
| US9018212B2 (en) | 2010-05-25 | 2015-04-28 | Boehringer Ingelheim International Gmbh | Pyridazine carboxamides as CCR2 receptor antagonists |
| US8962656B2 (en) | 2010-06-01 | 2015-02-24 | Boehringer Ingelheim International Gmbh | CCR2 antagonists |
| US10640506B2 (en) | 2010-11-19 | 2020-05-05 | Incyte Holdings Corporation | Cyclobutyl substituted pyrrolopyridine and pyrrolopyrimidines derivatives as JAK inhibitors |
| US9034884B2 (en) | 2010-11-19 | 2015-05-19 | Incyte Corporation | Heterocyclic-substituted pyrrolopyridines and pyrrolopyrimidines as JAK inhibitors |
| US8933085B2 (en) | 2010-11-19 | 2015-01-13 | Incyte Corporation | Cyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors |
| US8871774B2 (en) | 2010-12-16 | 2014-10-28 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| WO2012093169A1 (en) | 2011-01-07 | 2012-07-12 | Leo Pharma A/S | Novel sulfamide piperazine derivatives as protein tyrosine kinase inhibitors and pharmaceutical use thereof |
| US9233964B2 (en) | 2011-01-07 | 2016-01-12 | Leo Pharma A/S | Sulfamide piperazine derivatives as protein tyrosine kinase inhibitors and pharmaceutical use therof |
| US11337976B2 (en) | 2011-02-07 | 2022-05-24 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US9624213B2 (en) | 2011-02-07 | 2017-04-18 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US12076322B2 (en) | 2011-02-07 | 2024-09-03 | Plexxikon Inc. | Compounds and methods for kinase modulation, and indications therefor |
| US9993480B2 (en) | 2011-02-18 | 2018-06-12 | Novartis Pharma Ag | mTOR/JAK inhibitor combination therapy |
| US8865735B2 (en) | 2011-02-21 | 2014-10-21 | Hoffman-La Roche Inc. | Solid forms of a pharmaceutically active substance |
| US11214573B2 (en) | 2011-06-20 | 2022-01-04 | Incyte Holdings Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US9611269B2 (en) | 2011-06-20 | 2017-04-04 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US10513522B2 (en) | 2011-06-20 | 2019-12-24 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US8691807B2 (en) | 2011-06-20 | 2014-04-08 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US9023840B2 (en) | 2011-06-20 | 2015-05-05 | Incyte Corporation | Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors |
| US8946425B2 (en) | 2011-07-05 | 2015-02-03 | Vertex Pharmaceuticals Incorporated | Processes and intermediates for producing azaindoles |
| US8513414B2 (en) | 2011-07-05 | 2013-08-20 | Vertex Pharmaceuticals Incorporated | Processes and intermediates for producing azaindoles |
| US9090614B2 (en) | 2011-07-05 | 2015-07-28 | Vertex Pharmaceuticals Incorporated | Processes and intermediates for producing azaindoles |
| US8796453B2 (en) | 2011-07-05 | 2014-08-05 | Vertex Pharmaceuticals Incorporated | Processes and intermediates for producing azaindoles |
| US9108958B2 (en) | 2011-07-15 | 2015-08-18 | Boehringer Ingelheim International Gmbh | Selective CCR2 antagonists |
| US9394302B2 (en) | 2011-08-01 | 2016-07-19 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US9051319B2 (en) | 2011-08-01 | 2015-06-09 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US9908878B2 (en) | 2011-08-01 | 2018-03-06 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US10875855B2 (en) | 2011-08-01 | 2020-12-29 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US9358229B2 (en) | 2011-08-10 | 2016-06-07 | Novartis Pharma Ag | JAK PI3K/mTOR combination therapy |
| US9359358B2 (en) | 2011-08-18 | 2016-06-07 | Incyte Holdings Corporation | Cyclohexyl azetidine derivatives as JAK inhibitors |
| US9487521B2 (en) | 2011-09-07 | 2016-11-08 | Incyte Holdings Corporation | Processes and intermediates for making a JAK inhibitor |
| US9718834B2 (en) | 2011-09-07 | 2017-08-01 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US9663487B2 (en) | 2012-03-08 | 2017-05-30 | Karus Therapeutics Limited | Phosphoinositide 3-kinase inhibitors |
| WO2013132270A1 (en) * | 2012-03-08 | 2013-09-12 | Karus Therapeutics Limited | Phosphoinositide 3-kinase inhibitors |
| US10035785B2 (en) | 2012-03-08 | 2018-07-31 | Karus Therapeutics Limited | Phosphoinositide 3-kinase inhibitors |
| US10464940B2 (en) | 2012-03-29 | 2019-11-05 | GI Therapeutics, Inc. | Lactam kinase inhibitors |
| US9745316B2 (en) | 2012-03-29 | 2017-08-29 | G1 Therapeutics, Inc. | Lactam kinase inhibitors |
| WO2013148748A1 (en) * | 2012-03-29 | 2013-10-03 | Francis Xavier Tavares | Lactam kinase inhibitors |
| US9856268B2 (en) | 2012-03-29 | 2018-01-02 | G1 Therapeutics, Inc. | Lactam kinase inhibitors |
| US9260442B2 (en) | 2012-03-29 | 2016-02-16 | G1 Therapeutics, Inc. | Lactam kinase inhibitors |
| US9193733B2 (en) | 2012-05-18 | 2015-11-24 | Incyte Holdings Corporation | Piperidinylcyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors |
| US9695169B2 (en) | 2012-05-31 | 2017-07-04 | Plexxikon Inc. | Synthesis of heterocyclic compounds |
| US9150570B2 (en) | 2012-05-31 | 2015-10-06 | Plexxikon Inc. | Synthesis of heterocyclic compounds |
| US10150763B2 (en) | 2012-11-07 | 2018-12-11 | Karus Therapeutics Limited | Histone deacetylase inhibitors and their use in therapy |
| US9676765B2 (en) | 2012-11-07 | 2017-06-13 | Karus Therapeutics Limited | Histone deacetylase inhibitors and their use in therapy |
| US10166191B2 (en) | 2012-11-15 | 2019-01-01 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| US11896717B2 (en) | 2012-11-15 | 2024-02-13 | Incyte Holdings Corporation | Sustained-release dosage forms of ruxolitinib |
| US11576865B2 (en) | 2012-11-15 | 2023-02-14 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| US11337927B2 (en) | 2012-11-15 | 2022-05-24 | Incyte Holdings Corporation | Sustained-release dosage forms of ruxolitinib |
| US11576864B2 (en) | 2012-11-15 | 2023-02-14 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| US10874616B2 (en) | 2012-11-15 | 2020-12-29 | Incyte Corporation | Sustained-release dosage forms of ruxolitinib |
| FR3001219A1 (en) * | 2013-01-22 | 2014-07-25 | Centre Nat Rech Scient | KINASE INHIBITORS |
| WO2014115071A1 (en) * | 2013-01-22 | 2014-07-31 | Centre National De La Recherche Scientifique | Kinase inhibitors |
| US8987443B2 (en) | 2013-03-06 | 2015-03-24 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US9221845B2 (en) | 2013-03-06 | 2015-12-29 | Incyte Holdings Corporation | Processes and intermediates for making a JAK inhibitor |
| US9714233B2 (en) | 2013-03-06 | 2017-07-25 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| US9487530B2 (en) | 2013-03-15 | 2016-11-08 | G1 Therapeutics, Inc. | Transient protection of normal cells during chemotherapy |
| US10925878B2 (en) | 2013-03-15 | 2021-02-23 | G1 Therapeutics, Inc. | HSPC-sparing treatments for RB-positive abnormal cellular proliferation |
| US10660896B2 (en) | 2013-03-15 | 2020-05-26 | GI Therapeutics, Inc. | Transient protection of normal cells during chemotherapy |
| US9931345B2 (en) | 2013-03-15 | 2018-04-03 | Presidents And Fellows Of Harvard College | Transient protection of normal cells during chemotherapy |
| US11654148B2 (en) | 2013-03-15 | 2023-05-23 | G1 Therapeutics, Inc. | HSPC-sparing treatments for RB-positive abnormal cellular proliferation |
| US11040042B2 (en) | 2013-03-15 | 2021-06-22 | G1 Therapeutics, Inc. | Transient protection of normal cells during chemotherapy |
| US10966984B2 (en) | 2013-03-15 | 2021-04-06 | G1 Therapeutics, Inc. | Transient protection of normal cells during chemotherapy |
| US9527857B2 (en) | 2013-03-15 | 2016-12-27 | GI Therapeutics, Inc. | HSPC-sparing treatments for RB-positive abnormal cellular proliferation |
| US9464092B2 (en) | 2013-03-15 | 2016-10-11 | G1 Therapeutics, Inc. | Transient protection of normal cells during chemotherapy |
| US10076523B2 (en) | 2013-03-15 | 2018-09-18 | G1 Therapeutics, Inc. | HSPC-sparing treatments for RB-positive abnormal cellular proliferation |
| US11717523B2 (en) | 2013-03-15 | 2023-08-08 | G1 Therapeutics, Inc. | Transient protection of normal cells during chemotherapy |
| US10085992B2 (en) | 2013-03-15 | 2018-10-02 | G1 Therapeutics, Inc. | Transient protection of normal cells during chemotherapy |
| US10709711B2 (en) | 2013-03-15 | 2020-07-14 | G1 Therapeutics, Inc. | Highly active anti-neoplastic and anti-proliferative agents |
| US10434104B2 (en) | 2013-03-15 | 2019-10-08 | G1 Therapeutics, Inc. | HSPC-sparing treatments for Rb-positive abnormal cellular proliferation |
| US10870624B2 (en) | 2013-05-10 | 2020-12-22 | Karus Therapeutics Limited | Histone deacetylase inhibitors |
| US9862685B2 (en) | 2013-05-10 | 2018-01-09 | Karus Therapeutics Limited | Histone deacetylase inhibitors |
| US12151026B2 (en) | 2013-08-07 | 2024-11-26 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| US9655854B2 (en) | 2013-08-07 | 2017-05-23 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| US11045421B2 (en) | 2013-08-07 | 2021-06-29 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| US10561616B2 (en) | 2013-08-07 | 2020-02-18 | Incyte Corporation | Sustained release dosage forms for a JAK1 inhibitor |
| US10640501B2 (en) | 2013-11-13 | 2020-05-05 | Vertex Pharmaceuticals Incorporated | Methods of preparing inhibitors of influenza viruses replication |
| US11345700B2 (en) | 2013-11-13 | 2022-05-31 | Vertex Pharmaceuticals Incorporated | Methods of preparing inhibitors of influenza viruses replication |
| US9771361B2 (en) | 2013-11-13 | 2017-09-26 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US10023569B2 (en) | 2013-11-13 | 2018-07-17 | Vertex Pharmaceuticals Incorporated | Methods of preparing inhibitors of influenza viruses replication |
| US9782392B2 (en) | 2014-02-04 | 2017-10-10 | Taiho Pharmaceutical Co., Ltd. | Azaindole derivative |
| KR20160102568A (en) | 2014-02-04 | 2016-08-30 | 다이호야쿠힌고교 가부시키가이샤 | Azaindole derivative |
| US9676773B2 (en) | 2014-02-04 | 2017-06-13 | Taiho Pharmaceutical Co., Ltd. | Azaindole derivative |
| US9981987B2 (en) | 2014-02-12 | 2018-05-29 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US11708378B2 (en) | 2014-02-12 | 2023-07-25 | Convalife (Shanghai) Co. Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US10513530B2 (en) | 2014-02-12 | 2019-12-24 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US10059709B2 (en) | 2014-03-28 | 2018-08-28 | Elanco Tiergesundheit Ag | Compounds |
| US10370375B2 (en) | 2014-03-28 | 2019-08-06 | Elanco Tiergesundheit Ag | Heterocyclyl-substituted cyclohexylsulfonamides as JAK inhibitors |
| US10376519B2 (en) | 2014-04-17 | 2019-08-13 | G1 Therapeutics, Inc. | Tricyclic lactams for use in HSPC-sparing treatments for Rb-positive abnormal cellular proliferation |
| US9717735B2 (en) | 2014-04-17 | 2017-08-01 | G1 Therapeutics, Inc. | Tricyclic lactams for use in HSPC-sparing treatments for RB-positive abnormal cellular proliferation |
| US9498467B2 (en) | 2014-05-30 | 2016-11-22 | Incyte Corporation | Treatment of chronic neutrophilic leukemia (CNL) and atypical chronic myeloid leukemia (aCML) by inhibitors of JAK1 |
| CN107108581A (en) * | 2014-08-21 | 2017-08-29 | 百时美施贵宝公司 | It is used as the tieback heterocyclic carbamate derivatives of potent ROCK inhibitor |
| US10112939B2 (en) | 2014-08-21 | 2018-10-30 | Bristol-Myers Squibb Company | Tied-back benzamide derivatives as potent rock inhibitors |
| WO2016028971A1 (en) * | 2014-08-21 | 2016-02-25 | Bristol-Myers Squibb Company | Tied-back benzamide derivatives as potent rock inhibitors |
| US10413547B2 (en) | 2014-09-12 | 2019-09-17 | G1 Therapeutics, Inc. | Treatment of Rb-negative tumors using topoisomerase with cyclin dependent kinase 4/6 inhibitors |
| US11446295B2 (en) | 2014-09-12 | 2022-09-20 | G1 Therapeutics, Inc. | Anti-neoplastic combinations and dosing regimens using CDK4/6 inhibitor compounds to treat Rb-positive tumors |
| US11090306B2 (en) | 2014-09-12 | 2021-08-17 | G1 Therapeutics, Inc. | Treatment of Rb-negative tumors using topoisomerase inhibitors in combination with cyclin dependent kinase 4/6 inhibitors |
| US12285431B2 (en) | 2014-09-12 | 2025-04-29 | Pharmacosmos Holding A/S | Treatment of Rb-negative tumors using topoisomerase inhibitors in combination with cyclin dependent kinase 4/6 inhibitors |
| US10231969B2 (en) | 2014-09-12 | 2019-03-19 | GI Therapeutics, Inc. | Anti-neoplastic combinations and dosing regimens using CDK4/6 inhibitor compounds to treat RB-positive tumors |
| US10407435B2 (en) | 2014-10-29 | 2019-09-10 | Karus Therapeutics Limited | Diheteroaryl histone deacetylase inhibitors and their use in therapy |
| US10533003B2 (en) | 2014-10-29 | 2020-01-14 | Karus Therapeutics Limited | Polyheteroarl histone deacetylase inhibitors and their use in therapy |
| US10273233B2 (en) | 2015-05-13 | 2019-04-30 | Vertex Pharmaceuticals Incorporated | Inhibitors of influenza viruses replication |
| US10533004B2 (en) | 2015-05-13 | 2020-01-14 | Vertex Pharmaceuticals Incorporated | Methods of preparing inhibitors of influenza viruses replication |
| US10213428B2 (en) | 2015-07-02 | 2019-02-26 | Centrexion Therapeutics Corporation | (4-((3R,4R)-3-methoxytetrahydro-pyran-4-ylamino)piperidin-1-yl)(5-methyl-6-(((2R,6S)-6-(p-tolyl)tetrahydro-2H-pyran-2-yl)methylamino)pyrimidin-4-yl)methanone citrate |
| US10668077B2 (en) | 2015-08-19 | 2020-06-02 | Karus Therapeutics Limited | Compositions comprising phosphoinositide 3-kinase inhibitors and a second antiproliferative agent |
| US10377764B2 (en) | 2015-08-19 | 2019-08-13 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US11291669B2 (en) | 2015-08-19 | 2022-04-05 | Karus Therapeutics Limited | Compositions comprising phosphoinositide 3-kinase inhibitors and a second antiproliferative agent |
| US11779586B2 (en) | 2015-08-19 | 2023-10-10 | Convalife (Shanghai) Co. Limited | Compounds comprising tricyclic heterocyclic compounds |
| US10442815B2 (en) | 2015-08-19 | 2019-10-15 | Karus Therapeutics Limited | Tricyclic heterocyclic compounds as phosphoinositide 3-kinase inhibitors |
| US12257254B2 (en) | 2015-08-19 | 2025-03-25 | Convalife (Shanghai) Co. Limited | Compositions comprising phosphoinositide 3-kinase inhibitors and a second antiproliferative agent |
| RU2730849C1 (en) * | 2017-03-23 | 2020-08-26 | Даегу-Гиеонгбук Медикал Инновейшн Фаундейшн | Pyrrolo-pyridine compound derivative, method for production thereof and pharmaceutical composition containing same as active ingredient for preventing and treating protein kinase related diseases |
| US11117892B2 (en) | 2017-03-23 | 2021-09-14 | Daegu-Gyeongbuk Medical Innovation Foundation | Pyrrolo-pyridine derivative compound, method for preparing same, and pharmaceutical composition containing same as active ingredient for prevention or treatment of protein kinase-related diseases |
| US11390609B2 (en) | 2017-06-30 | 2022-07-19 | Beijing Tide Pharmaceutical Co., Ltd. | Rho-associated protein kinase inhibitor, pharmaceutical composition comprising same, and preparation method and use thereof |
| US10329282B2 (en) | 2017-06-30 | 2019-06-25 | Beijing Tide Pharmaceutical Co., Ltd. | Rho-associated protein kinase inhibitor, pharmaceutical composition comprising the same, as well as preparation method and use thereof |
| US10323023B2 (en) | 2017-06-30 | 2019-06-18 | Beijing Tide Pharmaceutical Co., Ltd. | Rho-associated protein kinase inhibitor, pharmaceutical composition comprising the same, as well as preparation method and use thereof |
| WO2019057103A1 (en) * | 2017-09-21 | 2019-03-28 | 上海华汇拓医药科技有限公司 | Jak inhibitor, preparation method therefor, and application in the field of medicine |
| US11278541B2 (en) | 2017-12-08 | 2022-03-22 | Incyte Corporation | Low dose combination therapy for treatment of myeloproliferative neoplasms |
| US10596161B2 (en) | 2017-12-08 | 2020-03-24 | Incyte Corporation | Low dose combination therapy for treatment of myeloproliferative neoplasms |
| US10899736B2 (en) | 2018-01-30 | 2021-01-26 | Incyte Corporation | Processes and intermediates for making a JAK inhibitor |
| WO2019179525A1 (en) * | 2018-03-23 | 2019-09-26 | Fochon Pharmaceuticals, Ltd. | Deuterated compounds as rock inhibitors |
| US11485707B2 (en) | 2018-03-23 | 2022-11-01 | Fochon Pharmaceuticals, Ltd. | Deuterated compounds as rock inhibitors |
| US11304949B2 (en) | 2018-03-30 | 2022-04-19 | Incyte Corporation | Treatment of hidradenitis suppurativa using JAK inhibitors |
| US12280054B2 (en) | 2018-03-30 | 2025-04-22 | Incyte Corporation | Treatment of hidradenitis suppurativa using JAK inhibitors |
| US12275731B2 (en) | 2018-10-16 | 2025-04-15 | Dana-Farber Cancer Institute, Inc. | Azaindole inhibitors of wild-type and mutant forms of LRRK2 |
| US12240844B2 (en) | 2019-01-18 | 2025-03-04 | Voronoi, Inc. | Pyrrolopyridine derivative and use thereof in prevention and treatment of protein kinase-related disease |
| WO2021011448A1 (en) * | 2019-07-12 | 2021-01-21 | The Johns Hopkins University | Compositions and methods for treating cerebral vasospasm |
| US11738026B2 (en) | 2019-11-22 | 2023-08-29 | Incyte Corporation | Combination therapy comprising an ALK2 inhibitor and a JAK2 inhibitor |
| US11833155B2 (en) | 2020-06-03 | 2023-12-05 | Incyte Corporation | Combination therapy for treatment of myeloproliferative neoplasms |
| US12168666B2 (en) | 2020-06-15 | 2024-12-17 | G1 Therapeutics, Inc. | Morphic forms of trilaciclib and methods of manufacture thereof |
| US10988479B1 (en) | 2020-06-15 | 2021-04-27 | G1 Therapeutics, Inc. | Morphic forms of trilaciclib and methods of manufacture thereof |
| US12194048B2 (en) | 2020-12-09 | 2025-01-14 | Voronoi Co., Ltd. | Use of pyrrolopyridine derivatives for preventing or treating inflammatory diseases |
| WO2023145372A1 (en) | 2022-01-28 | 2023-08-03 | 日本曹達株式会社 | Method for producing amide compound |
| KR20240142423A (en) | 2022-01-28 | 2024-09-30 | 닛뽕소다 가부시키가이샤 | Method for producing amide compounds |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20080016659A (en) | 2008-02-21 |
| US8921376B2 (en) | 2014-12-30 |
| CN101228161B (en) | 2012-10-10 |
| IL187438A0 (en) | 2008-02-09 |
| AU2006251623A1 (en) | 2006-11-30 |
| MX2007014619A (en) | 2009-02-13 |
| CN101228161A (en) | 2008-07-23 |
| RU2007147455A (en) | 2009-06-27 |
| RU2011133825A (en) | 2013-02-20 |
| EP1881983A1 (en) | 2008-01-30 |
| EP2354139A1 (en) | 2011-08-10 |
| ATE540948T1 (en) | 2012-01-15 |
| NZ564065A (en) | 2011-03-31 |
| CA2609126A1 (en) | 2006-11-30 |
| RU2435769C2 (en) | 2011-12-10 |
| EP2354140A1 (en) | 2011-08-10 |
| JP2009179644A (en) | 2009-08-13 |
| JP2008545660A (en) | 2008-12-18 |
| NO20076502L (en) | 2008-02-19 |
| JP2013253114A (en) | 2013-12-19 |
| EP1881983B1 (en) | 2012-01-11 |
| US20070135466A1 (en) | 2007-06-14 |
| ES2380795T3 (en) | 2012-05-18 |
| ZA200710379B (en) | 2009-05-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1881983B1 (en) | Pyrrolopyridines useful as inhibitors of protein kinase | |
| JP5144532B2 (en) | c-Met inhibitors and methods of use | |
| EP1844050B1 (en) | Pyrrolopyrimidines useful as inhibitors of protein kinase | |
| US20060122185A1 (en) | Bicyclic inhibitors of Rho kinase | |
| WO2007117494A1 (en) | Deazapurines useful as inhibitors of janus kinases | |
| EP2079740B1 (en) | Tricyclic heteroaryl compounds useful as inhibitors of janus kinase | |
| JP2008528705A5 (en) | ||
| WO2007041130A2 (en) | Deazapurines useful as inhibitors of janus kinases | |
| AU2008343173A1 (en) | Pyrazolo [1,5-a] pyrimidines useful as JAK2 inhibitors | |
| CA2681516A1 (en) | Compounds useful as inhibitors of janus kinases | |
| JP5112317B2 (en) | 3,5-Disubstituted pyrid-2-ones useful as inhibitors of the TEC family of non-receptor tyrosine kinases | |
| HK1122794A (en) | Pyrrolopyridines useful as inhibitors of protein kinase |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200680026391.8 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 187438 Country of ref document: IL |
|
| ENP | Entry into the national phase |
Ref document number: 2609126 Country of ref document: CA Ref document number: 2008512591 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 4479/KOLNP/2007 Country of ref document: IN Ref document number: MX/A/2007/014619 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006770824 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006251623 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 564065 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007147455 Country of ref document: RU Ref document number: 1020077029764 Country of ref document: KR |
|
| ENP | Entry into the national phase |
Ref document number: 2006251623 Country of ref document: AU Date of ref document: 20060522 Kind code of ref document: A |