WO2005112670A1 - Molecularly imprinted polymers selective for nitrosamines and methods of using the same - Google Patents
Molecularly imprinted polymers selective for nitrosamines and methods of using the same Download PDFInfo
- Publication number
- WO2005112670A1 WO2005112670A1 PCT/SE2005/000773 SE2005000773W WO2005112670A1 WO 2005112670 A1 WO2005112670 A1 WO 2005112670A1 SE 2005000773 W SE2005000773 W SE 2005000773W WO 2005112670 A1 WO2005112670 A1 WO 2005112670A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- molecularly imprinted
- tobacco
- imprinted polymer
- selective
- polymer
- Prior art date
Links
- 229920000344 molecularly imprinted polymer Polymers 0.000 title claims abstract description 106
- 238000000034 method Methods 0.000 title claims abstract description 53
- 150000004005 nitrosamines Chemical class 0.000 title claims abstract description 40
- 239000000463 material Substances 0.000 claims abstract description 84
- 235000019505 tobacco product Nutrition 0.000 claims abstract description 13
- 238000004519 manufacturing process Methods 0.000 claims abstract description 5
- 241000208125 Nicotiana Species 0.000 claims description 85
- 235000002637 Nicotiana tabacum Nutrition 0.000 claims description 85
- 150000001875 compounds Chemical class 0.000 claims description 51
- OGRXKBUCZFFSTL-UHFFFAOYSA-N 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol Chemical compound O=NN(C)CCCC(O)C1=CC=CN=C1 OGRXKBUCZFFSTL-UHFFFAOYSA-N 0.000 claims description 43
- 230000000391 smoking effect Effects 0.000 claims description 40
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 claims description 36
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 claims description 31
- 229960002715 nicotine Drugs 0.000 claims description 30
- 239000000779 smoke Substances 0.000 claims description 30
- 239000000178 monomer Substances 0.000 claims description 28
- 229920000642 polymer Polymers 0.000 claims description 27
- 239000000203 mixture Substances 0.000 claims description 22
- 125000000018 nitroso group Chemical group N(=O)* 0.000 claims description 17
- 238000006116 polymerization reaction Methods 0.000 claims description 15
- 238000005979 thermal decomposition reaction Methods 0.000 claims description 14
- 239000000284 extract Substances 0.000 claims description 13
- XKLJHFLUAHKGGU-UHFFFAOYSA-N nitrous amide Chemical compound ON=N XKLJHFLUAHKGGU-UHFFFAOYSA-N 0.000 claims description 13
- 239000002904 solvent Substances 0.000 claims description 10
- 238000002485 combustion reaction Methods 0.000 claims description 7
- VRWLBMGRSLQKSU-UHFFFAOYSA-N methanol;pyridine Chemical compound OC.C1=CC=NC=C1 VRWLBMGRSLQKSU-UHFFFAOYSA-N 0.000 claims description 7
- 239000004971 Cross linker Substances 0.000 claims description 5
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 claims description 5
- 239000003999 initiator Substances 0.000 claims description 5
- MTXSIJUGVMTTMU-JTQLQIEISA-N (S)-anabasine Chemical compound N1CCCC[C@H]1C1=CC=CN=C1 MTXSIJUGVMTTMU-JTQLQIEISA-N 0.000 claims description 4
- MYKUKUCHPMASKF-VIFPVBQESA-N (S)-nornicotine Chemical compound C1CCN[C@@H]1C1=CC=CN=C1 MYKUKUCHPMASKF-VIFPVBQESA-N 0.000 claims description 4
- SOPPBXUYQGUQHE-JTQLQIEISA-N Anatabine Chemical compound C1C=CCN[C@@H]1C1=CC=CN=C1 SOPPBXUYQGUQHE-JTQLQIEISA-N 0.000 claims description 4
- SOPPBXUYQGUQHE-UHFFFAOYSA-N Anatabine Natural products C1C=CCNC1C1=CC=CN=C1 SOPPBXUYQGUQHE-UHFFFAOYSA-N 0.000 claims description 4
- MYKUKUCHPMASKF-UHFFFAOYSA-N Nornicotine Natural products C1CCNC1C1=CC=CN=C1 MYKUKUCHPMASKF-UHFFFAOYSA-N 0.000 claims description 4
- 229930014345 anabasine Natural products 0.000 claims description 4
- 238000010438 heat treatment Methods 0.000 claims description 4
- 239000002245 particle Substances 0.000 claims description 4
- FXHQYUYOAGYBTE-UHFFFAOYSA-N 4-[methyl(prop-1-enyl)amino]-1-pyridin-3-ylbutan-1-ol Chemical compound CC=CN(C)CCCC(O)C1=CC=CN=C1 FXHQYUYOAGYBTE-UHFFFAOYSA-N 0.000 claims description 3
- 230000002378 acidificating effect Effects 0.000 claims description 3
- 150000003254 radicals Chemical group 0.000 claims description 3
- 239000012429 reaction media Substances 0.000 claims 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims 3
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 claims 1
- VLSRKCIBHNJFHA-UHFFFAOYSA-N 2-(trifluoromethyl)prop-2-enoic acid Chemical compound OC(=O)C(=C)C(F)(F)F VLSRKCIBHNJFHA-UHFFFAOYSA-N 0.000 claims 1
- XUDBVJCTLZTSDC-UHFFFAOYSA-N 2-ethenylbenzoic acid Chemical compound OC(=O)C1=CC=CC=C1C=C XUDBVJCTLZTSDC-UHFFFAOYSA-N 0.000 claims 1
- 238000011156 evaluation Methods 0.000 claims 1
- 125000005395 methacrylic acid group Chemical group 0.000 claims 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 claims 1
- 238000004458 analytical method Methods 0.000 abstract description 8
- 238000000926 separation method Methods 0.000 abstract description 7
- 239000013060 biological fluid Substances 0.000 abstract description 2
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 48
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 36
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 30
- 238000000605 extraction Methods 0.000 description 22
- 239000000243 solution Substances 0.000 description 22
- 239000000126 substance Substances 0.000 description 22
- 239000000047 product Substances 0.000 description 19
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 13
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 12
- 239000000523 sample Substances 0.000 description 12
- 235000019504 cigarettes Nutrition 0.000 description 10
- -1 nitrosamines Chemical class 0.000 description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- 239000012491 analyte Substances 0.000 description 9
- 150000002081 enamines Chemical class 0.000 description 9
- 210000002700 urine Anatomy 0.000 description 9
- 238000002414 normal-phase solid-phase extraction Methods 0.000 description 8
- 239000012071 phase Substances 0.000 description 8
- 230000008569 process Effects 0.000 description 8
- 238000011282 treatment Methods 0.000 description 8
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 8
- 229920002301 cellulose acetate Polymers 0.000 description 7
- 239000000123 paper Substances 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 239000001569 carbon dioxide Substances 0.000 description 6
- 229910002092 carbon dioxide Inorganic materials 0.000 description 6
- 238000010828 elution Methods 0.000 description 6
- 238000004128 high performance liquid chromatography Methods 0.000 description 6
- 238000011084 recovery Methods 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 5
- FLAQQSHRLBFIEZ-UHFFFAOYSA-N N-Methyl-N-nitroso-4-oxo-4-(3-pyridyl)butyl amine Chemical compound O=NN(C)CCCC(=O)C1=CC=CN=C1 FLAQQSHRLBFIEZ-UHFFFAOYSA-N 0.000 description 5
- 229960000583 acetic acid Drugs 0.000 description 5
- 238000009835 boiling Methods 0.000 description 5
- 230000001143 conditioned effect Effects 0.000 description 5
- 230000002452 interceptive effect Effects 0.000 description 5
- 239000011159 matrix material Substances 0.000 description 5
- 239000002207 metabolite Substances 0.000 description 5
- NBBJYMSMWIIQGU-UHFFFAOYSA-N Propionic aldehyde Chemical compound CCC=O NBBJYMSMWIIQGU-UHFFFAOYSA-N 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 229930013930 alkaloid Natural products 0.000 description 4
- 239000012153 distilled water Substances 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 239000001087 glyceryl triacetate Substances 0.000 description 4
- 235000013773 glyceryl triacetate Nutrition 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 238000012544 monitoring process Methods 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 238000011002 quantification Methods 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 239000002594 sorbent Substances 0.000 description 4
- 239000012086 standard solution Substances 0.000 description 4
- 229960002622 triacetin Drugs 0.000 description 4
- DBCAQXHNJOFNGC-UHFFFAOYSA-N 4-bromo-1,1,1-trifluorobutane Chemical compound FC(F)(F)CCCBr DBCAQXHNJOFNGC-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 3
- 239000000443 aerosol Substances 0.000 description 3
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 3
- 230000001055 chewing effect Effects 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- 230000003750 conditioning effect Effects 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- STVZJERGLQHEKB-UHFFFAOYSA-N ethylene glycol dimethacrylate Substances CC(=C)C(=O)OCCOC(=O)C(C)=C STVZJERGLQHEKB-UHFFFAOYSA-N 0.000 description 3
- 235000013305 food Nutrition 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 150000002832 nitroso derivatives Chemical class 0.000 description 3
- 230000000717 retained effect Effects 0.000 description 3
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 2
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 2
- BXYPVKMROLGXJI-JTQLQIEISA-N 3-[(2s)-1-nitrosopiperidin-2-yl]pyridine Chemical compound O=NN1CCCC[C@H]1C1=CC=CN=C1 BXYPVKMROLGXJI-JTQLQIEISA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- RYFOJXFXERAMLS-UHFFFAOYSA-N Nicotyrine Chemical compound CN1C=CC=C1C1=CC=CN=C1 RYFOJXFXERAMLS-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- 229910021536 Zeolite Inorganic materials 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 239000003463 adsorbent Substances 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 239000000538 analytical sample Substances 0.000 description 2
- 238000011953 bioanalysis Methods 0.000 description 2
- 239000012472 biological sample Substances 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- ZTQSAGDEMFDKMZ-UHFFFAOYSA-N butyric aldehyde Natural products CCCC=O ZTQSAGDEMFDKMZ-UHFFFAOYSA-N 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000013375 chromatographic separation Methods 0.000 description 2
- 235000019506 cigar Nutrition 0.000 description 2
- 239000012141 concentrate Substances 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 239000012362 glacial acetic acid Substances 0.000 description 2
- 230000009931 harmful effect Effects 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 239000003456 ion exchange resin Substances 0.000 description 2
- 229920003303 ion-exchange polymer Polymers 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 239000002808 molecular sieve Substances 0.000 description 2
- DPNGWXJMIILTBS-UHFFFAOYSA-N myosmine Chemical compound C1CCN=C1C1=CC=CN=C1 DPNGWXJMIILTBS-UHFFFAOYSA-N 0.000 description 2
- XMUAIHFHOPIFIJ-UHFFFAOYSA-N n-(1-hydroxy-1-pyridin-3-ylpentyl)nitrous amide Chemical compound CCCCC(O)(NN=O)C1=CC=CN=C1 XMUAIHFHOPIFIJ-UHFFFAOYSA-N 0.000 description 2
- XKABJYQDMJTNGQ-VIFPVBQESA-N n-nitrosonornicotine Chemical compound O=NN1CCC[C@H]1C1=CC=CN=C1 XKABJYQDMJTNGQ-VIFPVBQESA-N 0.000 description 2
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 239000011236 particulate material Substances 0.000 description 2
- 239000004014 plasticizer Substances 0.000 description 2
- 239000002861 polymer material Substances 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 239000003361 porogen Substances 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- SHNUBALDGXWUJI-UHFFFAOYSA-N pyridin-2-ylmethanol Chemical group OCC1=CC=CC=N1 SHNUBALDGXWUJI-UHFFFAOYSA-N 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 2
- 238000000935 solvent evaporation Methods 0.000 description 2
- 238000000638 solvent extraction Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 238000000194 supercritical-fluid extraction Methods 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 239000010457 zeolite Substances 0.000 description 2
- UIKROCXWUNQSPJ-VIFPVBQESA-N (-)-cotinine Chemical compound C1CC(=O)N(C)[C@@H]1C1=CC=CN=C1 UIKROCXWUNQSPJ-VIFPVBQESA-N 0.000 description 1
- GFXQUCWFEPCALC-UHFFFAOYSA-N 1-(4-isothiocyanato-2-nitrophenyl)imidazole Chemical compound [O-][N+](=O)C1=CC(N=C=S)=CC=C1N1C=NC=C1 GFXQUCWFEPCALC-UHFFFAOYSA-N 0.000 description 1
- WYGWHHGCAGTUCH-UHFFFAOYSA-N 2-[(2-cyano-4-methylpentan-2-yl)diazenyl]-2,4-dimethylpentanenitrile Chemical group CC(C)CC(C)(C#N)N=NC(C)(C#N)CC(C)C WYGWHHGCAGTUCH-UHFFFAOYSA-N 0.000 description 1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 1
- ZJOFAFWTOKDIFH-UHFFFAOYSA-N 3-(1-nitroso-3,6-dihydro-2h-pyridin-2-yl)pyridine Chemical compound O=NN1CC=CCC1C1=CC=CN=C1 ZJOFAFWTOKDIFH-UHFFFAOYSA-N 0.000 description 1
- ZJOFAFWTOKDIFH-JTQLQIEISA-N 3-[(2s)-1-nitroso-3,6-dihydro-2h-pyridin-2-yl]pyridine Chemical compound O=NN1CC=CC[C@H]1C1=CC=CN=C1 ZJOFAFWTOKDIFH-JTQLQIEISA-N 0.000 description 1
- NZSNJPDBPMIBSP-UHFFFAOYSA-N 4-[methyl(nitroso)amino]-4-pyridin-3-ylbutanoic acid Chemical compound OC(=O)CCC(N(N=O)C)C1=CC=CN=C1 NZSNJPDBPMIBSP-UHFFFAOYSA-N 0.000 description 1
- MAGFQRLKWCCTQJ-UHFFFAOYSA-N 4-ethenylbenzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=C(C=C)C=C1 MAGFQRLKWCCTQJ-UHFFFAOYSA-N 0.000 description 1
- IRQWEODKXLDORP-UHFFFAOYSA-N 4-ethenylbenzoic acid Chemical compound OC(=O)C1=CC=C(C=C)C=C1 IRQWEODKXLDORP-UHFFFAOYSA-N 0.000 description 1
- UIKROCXWUNQSPJ-UHFFFAOYSA-N Cotinine Natural products C1CC(=O)N(C)C1C1=CC=CN=C1 UIKROCXWUNQSPJ-UHFFFAOYSA-N 0.000 description 1
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 1
- 244000303040 Glycyrrhiza glabra Species 0.000 description 1
- 235000006200 Glycyrrhiza glabra Nutrition 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- YFCDLVPYFMHRQZ-UHFFFAOYSA-N N-Nitrosodiethanolamine Chemical compound OCCN(N=O)CCO YFCDLVPYFMHRQZ-UHFFFAOYSA-N 0.000 description 1
- WNYADZVDBIBLJJ-UHFFFAOYSA-N N-Nitrosopyrrolidine Chemical compound O=NN1CCCC1 WNYADZVDBIBLJJ-UHFFFAOYSA-N 0.000 description 1
- WBNQDOYYEUMPFS-UHFFFAOYSA-N N-nitrosodiethylamine Chemical compound CCN(CC)N=O WBNQDOYYEUMPFS-UHFFFAOYSA-N 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 150000003797 alkaloid derivatives Chemical class 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000000010 aprotic solvent Substances 0.000 description 1
- 239000012062 aqueous buffer Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 230000006399 behavior Effects 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229910010293 ceramic material Inorganic materials 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 229920001429 chelating resin Polymers 0.000 description 1
- 238000005557 chiral recognition Methods 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229950006073 cotinine Drugs 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000000378 dietary effect Effects 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 230000009881 electrostatic interaction Effects 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 238000010556 emulsion polymerization method Methods 0.000 description 1
- 238000003891 environmental analysis Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000013213 extrapolation Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 238000004186 food analysis Methods 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 238000001031 gas chromatography-thermal energy analyser Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 150000002303 glucose derivatives Chemical class 0.000 description 1
- LPLVUJXQOOQHMX-QWBHMCJMSA-N glycyrrhizinic acid Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@H](O[C@@H]1O[C@@H]1C([C@H]2[C@]([C@@H]3[C@@]([C@@]4(CC[C@@]5(C)CC[C@@](C)(C[C@H]5C4=CC3=O)C(O)=O)C)(C)CC2)(C)CC1)(C)C)C(O)=O)[C@@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O LPLVUJXQOOQHMX-QWBHMCJMSA-N 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 231100001261 hazardous Toxicity 0.000 description 1
- 239000000383 hazardous chemical Substances 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 150000007976 iminium ions Chemical class 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 235000011477 liquorice Nutrition 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical class C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 1
- 238000000874 microwave-assisted extraction Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- IIDMFFRDEFHWCJ-UHFFFAOYSA-N n-(4-hydroxy-1-pyridin-3-ylbutyl)-n-methylnitrous amide Chemical compound OCCCC(N(N=O)C)C1=CC=CN=C1 IIDMFFRDEFHWCJ-UHFFFAOYSA-N 0.000 description 1
- UMFJAHHVKNCGLG-UHFFFAOYSA-N n-Nitrosodimethylamine Chemical compound CN(C)N=O UMFJAHHVKNCGLG-UHFFFAOYSA-N 0.000 description 1
- FRJHUNPWTKLYGL-UHFFFAOYSA-N n-methyl-n-(4-oxo-1-pyridin-3-ylbutyl)nitrous amide Chemical compound O=CCCC(N(N=O)C)C1=CC=CN=C1 FRJHUNPWTKLYGL-UHFFFAOYSA-N 0.000 description 1
- 229940015769 nicotine chewing gum Drugs 0.000 description 1
- 239000000181 nicotinic agonist Substances 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- WLGDAKIJYPIYLR-UHFFFAOYSA-N octane-1-sulfonic acid Chemical compound CCCCCCCCS(O)(=O)=O WLGDAKIJYPIYLR-UHFFFAOYSA-N 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 239000000813 peptide hormone Substances 0.000 description 1
- 239000000575 pesticide Substances 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920002959 polymer blend Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000005588 protonation Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000000197 pyrolysis Methods 0.000 description 1
- 238000003908 quality control method Methods 0.000 description 1
- 238000010526 radical polymerization reaction Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000001338 self-assembly Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000007873 sieving Methods 0.000 description 1
- 230000005586 smoking cessation Effects 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000003270 steroid hormone Substances 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- 231100000606 suspected carcinogen Toxicity 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000010558 suspension polymerization method Methods 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- RWFBQHICRCUQJJ-JQWIXIFHSA-N trans-(S)-nicotine N(1')-oxide Chemical compound C[N@+]1([O-])CCC[C@H]1C1=CC=CN=C1 RWFBQHICRCUQJJ-JQWIXIFHSA-N 0.000 description 1
- 238000000825 ultraviolet detection Methods 0.000 description 1
- 239000005418 vegetable material Substances 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F226/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a single or double bond to nitrogen or by a heterocyclic ring containing nitrogen
- C08F226/06—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a single or double bond to nitrogen or by a heterocyclic ring containing nitrogen by a heterocyclic ring containing nitrogen
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/18—Treatment of tobacco products or tobacco substitutes
- A24B15/24—Treatment of tobacco products or tobacco substitutes by extraction; Tobacco extracts
- A24B15/241—Extraction of specific substances
- A24B15/245—Nitrosamines
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/18—Treatment of tobacco products or tobacco substitutes
- A24B15/24—Treatment of tobacco products or tobacco substitutes by extraction; Tobacco extracts
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24D—CIGARS; CIGARETTES; TOBACCO SMOKE FILTERS; MOUTHPIECES FOR CIGARS OR CIGARETTES; MANUFACTURE OF TOBACCO SMOKE FILTERS OR MOUTHPIECES
- A24D3/00—Tobacco smoke filters, e.g. filter-tips, filtering inserts; Filters specially adapted for simulated smoking devices; Mouthpieces for cigars or cigarettes
- A24D3/02—Manufacture of tobacco smoke filters
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24D—CIGARS; CIGARETTES; TOBACCO SMOKE FILTERS; MOUTHPIECES FOR CIGARS OR CIGARETTES; MANUFACTURE OF TOBACCO SMOKE FILTERS OR MOUTHPIECES
- A24D3/00—Tobacco smoke filters, e.g. filter-tips, filtering inserts; Filters specially adapted for simulated smoking devices; Mouthpieces for cigars or cigarettes
- A24D3/06—Use of materials for tobacco smoke filters
- A24D3/067—Use of materials for tobacco smoke filters characterised by functional properties
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24D—CIGARS; CIGARETTES; TOBACCO SMOKE FILTERS; MOUTHPIECES FOR CIGARS OR CIGARETTES; MANUFACTURE OF TOBACCO SMOKE FILTERS OR MOUTHPIECES
- A24D3/00—Tobacco smoke filters, e.g. filter-tips, filtering inserts; Filters specially adapted for simulated smoking devices; Mouthpieces for cigars or cigarettes
- A24D3/06—Use of materials for tobacco smoke filters
- A24D3/08—Use of materials for tobacco smoke filters of organic materials as carrier or major constituent
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24D—CIGARS; CIGARETTES; TOBACCO SMOKE FILTERS; MOUTHPIECES FOR CIGARS OR CIGARETTES; MANUFACTURE OF TOBACCO SMOKE FILTERS OR MOUTHPIECES
- A24D3/00—Tobacco smoke filters, e.g. filter-tips, filtering inserts; Filters specially adapted for simulated smoking devices; Mouthpieces for cigars or cigarettes
- A24D3/06—Use of materials for tobacco smoke filters
- A24D3/12—Use of materials for tobacco smoke filters of ion exchange materials
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/38—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving specific interaction not covered by one or more of groups B01D15/265 and B01D15/30 - B01D15/36, e.g. affinity, ligand exchange or chiral chromatography
- B01D15/3852—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving specific interaction not covered by one or more of groups B01D15/265 and B01D15/30 - B01D15/36, e.g. affinity, ligand exchange or chiral chromatography using imprinted phases or molecular recognition
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/22—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising organic material
- B01J20/26—Synthetic macromolecular compounds
- B01J20/268—Polymers created by use of a template, e.g. molecularly imprinted polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F236/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, at least one having two or more carbon-to-carbon double bonds
- C08F236/02—Copolymers of compounds having one or more unsaturated aliphatic radicals, at least one having two or more carbon-to-carbon double bonds the radical having only two carbon-to-carbon double bonds
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/0098—Plants or trees
Definitions
- the present invention relates to a class of molecularly imprinted polymers and use of the molecularly imprinted polymers in bioanalysis and separation of nicotine metabolites.
- the invention further relates to methods of using the molecularly imprinted polymers to treat tobacco, tobacco substitutes, and their derivatives to reduce the level of targeted compounds therein.
- the aim can be the quantitative extraction of a certain compound or compounds, the measurement of their concentration or the selective removal of a target compound from a multi-component mixture.
- nitrosamine nicotine metabolites may be produced in vivo by natural metabolic processes during the residence of the nicotine within body tissues. The levels of these metabolites remain below the concentrations at which most analytical procedures can perform quantitatively. The need for methods
- Targeted compounds for quantification, reduction or removal from tobacco or smoke are :0 known and include the major components of tobacco-specific nitrosamines (TSNAs) and their alkaloid precursors: NNK, 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone; NNA, 4-(methylnitrosamino)-4-(3-pyridyl)butanal; NNN, N-nitrosonornicotine; NAB, N- nitrosoanabasine; NAT, N-nitrosoanatabine; NNAL, 4-(methylnitrosamino)-l-(3- pyridy ⁇ )-l-butanol; iso-NNAL, 4-(methylnitrosamino)-4-(3-pyridyl)-l-butanol; iso-5 NNAC, 4-(methylnitrosamino)-4-(3-pyridyl)butanoic acid.
- TSNAs tobacco-specific
- a typical procedure might involve up to seven work-up steps including centrifugations, pH adjustments, enzymatic treatments, etc., which may sum up to a preparation time of many hours or even days per sample.
- loss of material during the process can lead to errors in estimation of the original sample concentrations, requiring extrapolation back from the final measurement, rather then reliance on direct measurement, to obtain the original concentration in the sample.
- a quick and simple method for the analysis of tobacco-specific nitrosamines is therefore a significant unmet medical analytical need. (See, e.g. Byrd & Ogden, Journal of Mass Spectrometry, 2003, 38, 98-107 and Wu et al. Anal.Chem. 2003, 75, 4827-4832).
- MIPs molecular imprinted polymers
- SPE solid-phase extraction
- MISPE Molecularly imprinted solid phase extractions
- MIPs have attracted considerable interest from the food > industry as a tool to improve food quality. This requires the use of a MIP for selective removal of undesirable components from the food matrix. Since these components are often present in low concentrations, the saturation capacity of the MIP is typically not a limiting factor.
- the preferred specifically designed MIP material of the invention is capable of selectively absorbing the most common nitrosylated nicotine derivatives from complex matrices, such as urine, giving quantitative recovery and thereby leading to low errors in the estimation of such hazardous chemical concentrations.
- the protein content of tobacco material is reduced by treating the tobacco with a solution containing a surfactant to extract polypeptides, separating the solution, removing the surfactant and the polypeptides from the solution, and recombining the solution with the tobacco material.
- International patent specification WO 01/65954 discloses a process in which tobacco is contacted with a supercritical extraction fluid such as supercritical
- the present invention provides a molecularly imprinted polymer (MIP) selective for nitroso-containing compounds.
- MIPs of the invention are selective for nitrosamines, in particular TSNAs or the volatile nitrosamines found in the vapour phase of the thermal decomposition products of smoking materials.
- Another preferred MIP of the invention is selective for
- nitrosylated derivatives of nicotine or the other alkaloids found in tobacco namely nornicotine, anabasine and anatabine.
- the MIPs of the invention can be obtained, for example, by co-polymerising a functional monomer, or monomers and a cross-linker in the presence of a structural analogue of a ) nitrosamine, in a polymerization medium containing a free radical initiator, after which the template is removed from the MIP.
- the invention includes the use of the molecularly-imprinted polymers of the invention for analytical and preparative extractions, in chromatography, for analytical sample pre-
- the invention includes a method of reducing the level of a targeted component in a tobacco product, in which the tobacco product is treated with a MIP ) which is selective for at least one nitroso-containing compound. Further, the invention provides methods of manufacturing a smoking material which incorporates use of MIPs to selectively remove nitroso-containing compounds.
- the present invention includes the treatment of tobacco products with MIPs to reduce the
- tobacco product means a material containing tobacco (including tobacco leaf or tobacco stem), or a tobacco substitute, or a blend of tobacco and tobacco substitutes, and derivatives of such material, including extracts of the material, smoke ) produced by thermal decomposition of the material and aerosols produced by heating the material to below its combustion temperature.
- tobacco product is a derivative produced by the thermal decomposition of material containing tobacco or a tobacco substitute
- the decomposition may be effected by combustion of the material, as in a conventional cigarette, or by heating the material to a temperature below its combustion temperature, in accordance with a process used in some known alternative tobacco products in order to produce an aerosol that is inhaled by the consumer.
- the tobacco product may be a derivative produced by contacting material containing tobacco or a tobacco substitute with a solvent.
- the invention provides a method of manufacturing a material for smoking comprising the steps of extracting smokable material with a solvent, treating the extract with a molecularly imprinted polymer selective for at least one nitroso-compound to reduce the level thereof in the extract and combining the treated extract with the smokable material.
- the smokable material may be in any convenient form, for example fines, stems, scraps, cut lamina, shredded stems, or any combination thereof.
- the solvent may be aqueous or non-aqueous, such as methanol, ethanol or a super-critical fluid extraction medium, such as super-critical carbon dioxide liquid.
- the extraction may be carried out under any conditions favoring the extraction of nitrogen-containing compounds from tobacco.
- the invention also includes a smoking article comprising tobacco or tobacco substitute, and a molecularly imprinted polymer selective for the removal of at least one nitroso- containing compound from the thermal decomposition product thereof.
- the smoking article of the invention may take any conventional form, for example a cigarette, cigar or cigarillo.
- the smoking article may comprise a rod of smoking material optionally in a wrapper, with or without a filter.
- the wrapper may be of paper, tobacco leaf, reconstituted tobacco or a tobacco substitute.
- the wrapper may be composed of non-combustible inorganic material such as a ceramic material.
- the filter may be of any suitable material, for example fibrous cellulose acetate, polypropylene or polyethylene, or paper.
- the smoking material is preferably tobacco but may be a tobacco substitute such as non- tobacco smoking material.
- non-tobacco smoking materials are dried and cured vegetable material, including fruit materials, and a synthetic smoking material such as may be produced from alginates and an aerosol-generating substance such as glycerol.
- the smoking material may also comprise a blend of tobacco and non-tobacco smoking materials.
- the tobacco may of any suitable type, or a blend thereof, including air-cured, fire-cured, flue-cured, or sun-cured lamina or stem, and may have been processed using any appropriate process.
- the tobacco may be cut, shredded, expanded or reconstituted.
- the smoking material may also include conventional additives, such as ameliorants, colorants, humectants (such as glycerol and propylene glycol), inert fillers (such as chalk), and flavourings (such as sugar, liquorice and cocoa).
- ameliorants such as glycerol and propylene glycol
- humectants such as glycerol and propylene glycol
- inert fillers such as chalk
- flavourings such as sugar, liquorice and cocoa
- the invention may also be applied to tobacco that is intended for oral or nasal consumption by sucking, chewing, or nasal ingestion, rather than smoking.
- tobacco that is intended for oral or nasal consumption by sucking, chewing, or nasal ingestion, rather than smoking.
- Such products include snuff, snus and "hard” or chewing tobacco.
- the molecularly imprinted material may be incorporated in the smokable material. Accordingly, the invention includes smoking material containing a molecularly imprinted polymer selective for the removal of at least one nitroso-containing compound from the thermal decomposition products of the smokable material.
- the molecularly imprinted material may be incorporated in the wrapper.
- the invention therefore includes wrapper material for smoking articles comprising a molecularly-imprinted polymer selective for the removal of a targeted component from the thermal decomposition products of a smoking material.
- the wrapper may be a cellulose-based material such a paper or a tobacco based material such as reconstituted tobacco.
- the preferred smoking articles of the invention are cigarettes, comprising a rod of tobacco, wrapper, and a filter including a molecularly imprinted polymer selective for the removal of at least one nitroso-containing compound from the thermal decomposition products of a smokable material.
- the invention also includes a smoke filter comprising a molecularly imprinted polymer selective for the removal of at least one nitroso-containing compound from the thermal decomposition products of a smoking material.
- the smoke filter may be produced separately from the smoking article, for example in the form of a cigarette or cigar holder, or it may be integrated into the smoking article, for example in the form of a i cigarette with a filter tip.
- Smoke filters in the form of filter tips may be of any conventional construction.
- it may in the form of a "dalmatian” type filter comprising a section of fibrous filter material, such as cellulose acetate, the molecularly imprinted polymer being in i particulate form and distributed throughout the section.
- the filter may be in the form of a "cavity" type filter, comprising multiple sections wherein the molecularly imprinted polymer may lie between two adjacent sections of fibrous filter material.
- the smoke filter may also comprise other adsorbent materials such as an ion-exchange resin, a zeolite, silica, alumina or amberlite.
- the smoke passes through the filter, the molecularly imprinted polymer selectively adsorbs and retains the targeted compounds from the smoke and the filtered smoke is delivered to the smoker.
- the smoke filters and smoking articles according to the invention may include means for protecting the molecularly imprinted polymer from, or reducing its exposure to, smoke when in use.
- the smoke filter may comprise a filter element for adsorbing materials from the vapour or particulate phase of smoke.
- Such filter elements may comprise a general adsorbent such as activated carbon, which may be in any convenient form, such as threads, particles, granules, cloth, or paper.
- the filter element may also be a selective adsorbant such as an ion-exchange resin, a zeolite, silica, alumina or amerlite.
- the means for protecting the catalyst may include two or more such filter elements of different compositions, for example a first filter element of cellulose acetate, and a second filter element of activated carbon.
- a first filter element of cellulose acetate for example a first filter element of cellulose acetate
- a second filter element of activated carbon for example a second filter element of activated carbon.
- Figure 1 shows an outline of the procedure for synthesis of an imprinted polymer
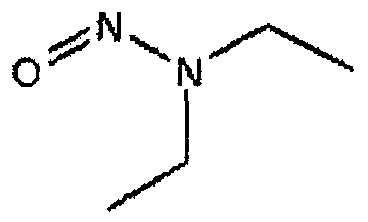
- Figure 2 shows the nitrosamine functional group and examples of nicotine related nitrosamine targets
- Figure 3 shows isosteric analogues of nitrosamines
- Figure 4A shows examples of amide and sulfonamide based target analogs
- Figure 4B shows an enamine target analogue (MPAPB) used as a template to prepare a MIP for extraction of NNAL;
- MPAPB enamine target analogue
- Figure 4C shows pyridine carbinol used as a template to prepare a MIP for extraction of NNAL
- Figure 5 shows recovery rates of NNAL using an NNAL-selective MIP
- Figure 6 shows chromatograms obtained after analysis of 1 mL human urine spiked with 0.25 ⁇ g NNAL (represented by solid line) and 1 mL blank human urine (representec by bold line);
- Figure 7 shows an overlay of chromatograms obtained after sample analysis in the presence of nicotine where the solid line represents NNAL and nicotine-spiked sample, dashed line represents eluent collected from loading 1 mL of the NNAL and nicotine- spiked sample, and the long dashed line represents a wash of (NH 4 )H 2 P0 4 , pH4.5;
- Figure 8 is a side elevation, partly longitudinal cross-section and partially broken away view of a smoking article with a smoke filter according to the invention;
- Figure 9 is a similar view to Figure 8 of a smoking article with an alternative smoke filter according to the invention;
- Figure 10 shows the chemical structure and boiling point for three products described in Example 6; and
- Figure 1 1 shows the chemical structure and boiling point for select volatile nitrosamines.
- Molecular imprinting typically consists of the following steps: (1) a template compound, which may be the targeted molecule or a structural analogue thereof, is allowed to interact with a selected functional monomer, or monomers, in solution to form a template-monomer complex; (2) the template-monomer complex is co-polymerized with a cross-linking monomer resulting in a polymeric matrix incorporating the template compound; (3) the template compound is extracted from the polymer matrix to form a MIP that can be used for selective binding of the targeted molecule. Prior to step (3), where the MIP is prepared as a solid polymer (or monolith) it is typically crushed and sieved to obtain a desired size fraction of particulate material.
- Particulate material prepared by any of the aforementioned methods can be packed into a chromatographic or solid phase extraction column and used for chromatographic separation of the template from other components of a mixture, including molecules with similar structures or functionalities.
- the reactive sites on the molecularly imprinted polymer exposed by removal of the template compound will be in a stereo-chemical configuration appropriate for reaction with fresh molecules of the targeted molecule.
- the polymer can be used for selective binding of the targeted molecule.
- the polymerization is performed in the presence of a pore-forming solvent called a porogen.
- a porogen is often chosen from among aprotic solvents of low to moderate polarity.
- template compounds exhibit moderate to high solubility in the polymerization media and these, or their structural analogues, can therefore be used directly using this standard procedure.
- the targeted molecule itself as the template, a structural analog of the target molecule is commonly preferred because: (a) the targeted molecule may be unstable under the polymerization conditions or may inhibit the polymerization; (b) the targeted molecule may not be available in sufficient quantities due to complexity of its synthesis or cost, or both; (c) the template may be insoluble or poorly soluble in the pre- polymerization mixture; (d) the MIP may remain contaminated by low levels of the targeted molecule retained in poorly accessible regions of the polymer matrix, which may bleed from the MIP during use; and/or (e) the target analyte(s) may present a significant health risk and should not be used as a template(s).
- TSNAs nitroso-compounds
- functional analogues thereof for example, glucose derivatives of TSNAs may be particularly useful as template compounds, see Figure 2.
- the functional analogue should be isosteric and preferably also isoelectronic with the targeted compound, or it may contain a substructure of the targeted compound where strong interactions may be likely.
- TSNAs tobacco-specific nitrosamines
- NNK 4-(methylnitrosamino)- 1 -(3-pyridyl)- 1 -butanone
- NNN N'-nitrosonornicotine
- N'-nitrosoanabasine N'-nitrosoanabasine
- NAT N'-nitrosoanatabine
- NNA 4-(methylnitrosamino)-4-(3-pyridyl)butanol
- N'-nitrosodimethylamine (“NDMA”)
- NDEA N'-nitrosodiethylamine
- nitroso-containing compounds have also been identified in chemical studies of tobacco or tobacco smoke, for example:
- N-nitrosopyrrolidine (“NYPR”):
- BMNA N'-nitrosomethylbutylnitrosamine
- NBA N'-nitroso-n-butylamine
- NIPI N'-nitrosopiperidine
- a particularly interesting template was identified, corresponding to the pyridine carbinol substructure but surprisingly lacking the nitrosamine moiety ( Figure 4B). If sufficient binding affinity and selectivity can be obtained for such sub-structural templates, this is the preferred approach. In fact, the binding affinity, selectivity and recoveries obtained with this pyridine carbinol MIP are superior to the MIPs obtained with the more complex enamine template.
- the invention provides a surprisingly effective MIP which comprises a simple template lacking certain key features of the target but providing for 5 effective binding with those target nitrosamines which contain the pyridine-methanol moiety.
- This invention includes an extraction method for quantitative recovery of the nicotine analog NNAL that entails the steps of preparation of an NNAL-selective MIP in a chromatographic material format, column conditioning, application of a urine sample, 0 removal of interfering compounds and finally selective elution of the NNAL analyte.
- the invention refers to template molecules, polymer materials designed to bind nitrosamines deriving from nicotine and '.5 present in organic or aqueous systems, and finally use of said materials in, for example, analytical or preparative separations, in chromatography, for analytical sample pre- treatment, and in chemical sensors.
- MIP was coarsely crushed and transferred to a Soxhlet thimble. It was extensively washed first with methanol for 12 hours and then with acetic acid for 12 hours in order to remove any remaining template and other non-reacted monomers. After these first extraction steps, the polymer was vacuum dried and then ground and sieved to a fine powder within a size range of 20 to 90 ⁇ m. As a final extraction step, the finely ground MIP was subjected to a 40 minutes microwave assisted solvent extraction using formic acid as the extraction solution. After drying, the MIP was ready for use.
- Example 3 Use of MIPs as selective sorbents in SPE
- the MIP can be packed into solid phase extraction columns for the selective extraction of NNAL from a biological matrix.
- a polypropylene frit was placed in an appropriate SPE column (typically 10ml capacity for analytical uses), 25 mg of the MIP was then added on top to form a MIP bed and the second frit was firmly pressed onto the surface of the MIP bed.
- Conditioning of the column was carried out in the following order: 1 ml DCM, 1 ml MeOH and finally 1 ml distilled water were added to the MIPSPE.
- the sample e.g. human urine (5 mL) containing low amounts of the analyte was allowed to pass through the conditioned MIPSPE column.
- the column was then subjected to vacuum in order to remove the water until the material was dry.
- polar interfering substances that may have non-specifically associated with the MIP were eluted by a wash with 1 ml distilled water. Again, a drying step using several minutes of vacuum was performed in order to enable the so-called phase-switch (change of the environment from aqueous to organic).
- phase-switch change of the environment from aqueous to organic.
- non-polar interfering substances were removed by washes with each 1 ml toluene, toluene:DCM (9:1) and toluene:DCM (4:1).
- the final selective elution of NNAL was carried out in 3 times elution steps, each of 1 ml DCM.
- the samples were reconstituted in the mobile phase and analyzed on an HPLC system: e.g. Merck-Hitachi (L-7000 system) using a beta-basic C18 column, 5 ⁇ m, 150x2.1 mm + pre-column 10x2.1 mm. Flow was at 0.25 mL/min, injection volume 100 ⁇ L, temperature 30°C and detection at UV 262 run.
- the mobile phase consists of 50 mM NH P04 pH 3, 5mM octanesulfonic acid and 20 % methanol.
- NNAL was obtained as a clearly distinguishable double peak eluting at about 8-10 minutes (see Figure 6, where a 1ml sample of human urine spiked with 0.25 ⁇ g NNAL is compared with NNAL-free urine).
- the double peak is characteristic for NNAL as it corresponds to its two rotamers. From the structure of NNAL, it can be demonstrated that the side chain on the pyridine ring can have different conformational states. The preferred conformations are called rotamers and for NNAL there are two major conformations. The retention of these two rotamers on an HPLC column will differ.
- the NNAL peak is cleanly separated from interfering substances. It can therefore be easily and accurately quantified.
- Recovery rates for NNAL are typically up to 90%, depending on the initial levels of NNAL in the biological sample. Recovery rates of close to 100% have been seen with samples containing 50pg/ml and 500pg/ml of NNAL ( Figure 5).
- Example 4 Use of MIPs as selective sorbents in SPE in the presence of Nicotine
- Another application of the invention is the use of the MIP as a selective sorbent for NNAL where there are high levels of nicotine present. This illustrates the wide scope of applications of the MIP material and how the selective nature of the MIP can be fine- tuned for particular samples.
- SPE columns were prepared as described in Example 3. Conditioning of the SPE column was carried out in the following order: 1 ml DCM followed by 1 ml MeOH followed by 1 ml 50 mM (NH 4 )H 2 P0 4 , pH 4.5.
- the sample in this example 5 mL human urine containing low amounts of the analyte was allowed to pass through the conditioned MIPSPE column.
- the column was then subjected to a mild vacuum (e.g., 10-80k Pa) to remove water until the material was dry.
- a mild vacuum e.g., 10-80k Pa
- Polar interfering substances that may have non- specifically associated with the MIP were eluted by a wash with 1 ml 50mM (NH 4 )H 2 P0 , pH 4.5. Another drying step of several minutes of mild vacuum was performed.
- Example 5 Smoking Articles incorporating MIPs
- Figures 8 and 9 illustrate smoking articles in the form of cigarettes having a rod 1 of tobacco encased in a wrapper 2 attached to a smoke filter 3 by means of a tipping paper 4.
- the tipping paper 4 is shown spaced from the wrapper 2, but in fact they will lie in close contact.
- the smoke filter 3 comprises three cylindrical filter elements 3a, 3b, 3c.
- the first filter element 3a at the mouth end of the filter is 7mm in length, composed of cellulose acetate tow impregnated with 7% by weight of triacetin plasticizer having a 25mm water gauge pressure drop over its length.
- the second filter element 3b positioned centrally is a cavity 5mm in length containing 150 mg of activated carbon granules.
- the third filter element 3c adjacent the rod 1 is 15 mm in length, has a 90 mm water gauge pressure drop over its length, and comprises 80mg cellulose acetate tow.
- the tow is impregnated with 4% by weight of triacetin and has 80mg of MIP specific for volatile nitrosamines, produced as described in Example 6 below, distributed evenly throughout its volume in a "Dalmatian" style.
- the cigarette shown in Figure 9 is similar to that of Figure 8 except that the smoke filter 3 has four coaxial, cylindrical filter elements 3a, 3b, 3c and 3d.
- the first filter element 3a at the mouth end of the cigarette is 5mm in length, and composed of cellulose acetate tow impregnated with 7% by weight of triacetin plasticizer.
- the second filter element 3b, positioned adjacent the first filter element 3a is a cavity 5mm in length containing 200 mg of molecularly-imprinted polymer specific for volatile nitrosamines, produced as described in Example 6 below.
- the third filter element 3c adjacent the second filter element 3b is 10 mm in length and comprises cellulose acetate tow impregnated with 7% by weight of triacetin.
- the fourth filter element 3d lies between the third filter element 3c, is 7mm in length and comprises 80mg of granular activated carbon.
- a ring of ventilation holes 5 is formed in the tipping paper 4 in a radial plane A-A which deliver air into the third filter element 3c about 3 mm downstream of the junction with the fourth filter
- the product is obtained in approximately 50% yield by distillation of the filtrate under reduced pressure, depending on the boiling point of the product.
- structures and boiling points are shown in Figure 10. (See, Brannock, et. al., J. Org. Chem., 1964, 29, 801-812.)
- the enamine is protonated, thus creating the necessary non-covalent interaction during the imprinting step.
- the positive charge resides on the carbon atom attached to the nitrogen, a structure stabilized due to delocalization to give an iminium ion. This positions the acidic functional monomers correctly for later recognition of volatile nitrosamines. As there is no opportunity to delocalise the positive charge, protonation of the enamine nitrogen is disfavored. (See, Cook, et al., J. Org. Chem., 1995, 60, 3169-3171.)
- a pre-polymerization solution is prepared by dissolving the desired enamine (1 mmol), an acidic functional monomer (4 mmol), a cross-linking monomer (20 mmol) and a free- radical initiator (1% w/w total monomers) in an appropriate porogenic solvent.
- the functional monomer is either MAA or trifiuoromethacrylic acid (TFMAA)
- the cross- linker is either EDMA or TRIM
- the free-radical initiator is ABDV
- the porogenic i solvent is one of chloroform, toluene, acetonitrile or acetonitrile/toluene (1/1 v/v).
- the solution is transferred to a polymerization vessel, cooled to 0°C and then purged with N 2 for 5 minutes, after which the vessel is flame sealed. Polymerization is initiated at 45°C and allowed to continue at this temperature for 24 hours. The polymer is then cured at 70 °C for a further 24 hours.
- the crude MIP material is then processed.
- the MIP is coarsely crushed and transferred to a Soxhlet thimble. It is then extensively extracted (i) with methanol for 12 hours and (ii) with acetic acid for 12 hours, in order to remove the template molecule and any unreacted monomers.
- the polymer is vacuum dried, ground, and sieved to give particles of the desired size range, e.g. 25-36 ⁇ m.
- the finely-ground MIP is then subjected to a final extraction step, involving 40 minutes microwave assisted extraction using formic acid as the extraction solvent.
- the MIP is then dried in vacuo for 24 hours.
- Example 8 Use of the MIP material of Example 2 and/or Example 7 in the treatment of tobacco extracts
- the polymer produced in accordance with the method of Example 2 or Example 7 is incorporated into a solid phase extraction column, and the column is conditioned by passing through dichloromethane (DCM), methanol and finally distilled water.
- DCM dichloromethane
- Shredded Burley tobacco leaf is extracted with water for 15 minutes at 60°C.
- the tobacco 3 is separated from the solution by filtration and dried.
- the solution is passed through the column and allowed to adsorb TSNA from the extract.
- the column is then drained and the solution concentrated by film evaporation, the concentrate is then recombined with the extracted tobacco and dried in air.
- TSNA adsorbed by the polymer can be eluted from the column using DCM.
- Example 9 Use of the MIP material of Example 2 or Example 7 in the treatment of tobacco extracts
- Flue-cured shredded tobacco leaf is extracted with water for 15 minutes at 60°C.
- the tobacco is separated from the solution by filtration and dried.
- the solution is mixed with the MIP of Example 2 or Example 7, during which period the polymer adsorbs the TSNAs selectively from the solution.
- the MIP is then mechanically separated from the extract by filtration or by centrifugation.
- the solution is concentrated by evaporation; the concentrate is then recombined with the extracted tobacco and dried in air.
- the MIP can be regenerated by elution with DCM, methanol and finally deionised water or pH 4 buffer, for reuse.
- Example 10 Use of the MIP material of Example 2 or Example 7 in the treatment of tobacco extracts Using a continuous extraction process, US Blend-type shredded tobacco leaf is loaded into a first extraction chamber into which super-critical carbon dioxide is fed. After contacting the tobacco, the carbon dioxide is fed into a second extraction chamber
- Example 5 containing a MIP produced as described in Example 2 or Example 7. Having contacted the polymer, the carbon dioxide is returned to the first extraction chamber and contacted again with the tobacco. The cyclic process is continued until the TSNA content of the tobacco has been reduced to a desired level, whereupon the carbon dioxide is vented from the system, and the tobacco removed from the first chamber.
- Example 11 Use of the molecularly-imprinted polymer material developed for 4- methylnitrosoamino-l-(3-pyridyl)-l-butanol (NNAL), in the treatment of an NNAL and nicotine containing solution i
- the polymer produced in accordance with the method of Example 2 was incorporated into a solid phase extraction column, and the column was conditioned by passing through phosphate buffer solution.
- Aqueous standard solutions of NNAL and nicotine were prepared in phosphate buffers over the pH range 3.0 - 7.5.
- the buffered standard solution was passed through the column, this fraction was collected and analyzed for NNAL and nicotine content.
- a buffered wash solution was passed through the column, this fraction was also collected and analyzed for NNAL and nicotine content.
- Example 12 Use of the MIP material developed for 4-methylnitrosoamino-l-(3-pyridyl)- 1-butanol (NNAL), in the treatment of a NNAL and TSNA containing solution
- the polymer produced in accordance with the method of Example 2 is incorporated into 5 a solid phase extraction column, and the column was conditioned by passing through dichloromethane (DCM), methanol and finally distilled water.
- DCM dichloromethane
- Aqueous standard solutions of NNAL and TSNAs were acidified with glacial acetic acid to pH 3.
- the standard solution was passed through the ) column, followed by three glacial acetic acid solution washes, this fraction was analyzed for NNAL and TSNA content by GC-TEA.
- Three washes of dichloromethane were passed through the column, this fraction was also analyzed for NNAL and TSNA content.
- the MIP retained 91% of the NNAL, 65% of the NNK and an efficiency of about 20- i 30% for the other (less structurally similar) TSNAs.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Analytical Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Polymers & Plastics (AREA)
- Biochemistry (AREA)
- Food Science & Technology (AREA)
- Physics & Mathematics (AREA)
- Wood Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Botany (AREA)
- Plural Heterocyclic Compounds (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Cigarettes, Filters, And Manufacturing Of Filters (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
- Treatment Of Liquids With Adsorbents In General (AREA)
- Polyesters Or Polycarbonates (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Polyamides (AREA)
Abstract
Description
Claims
Priority Applications (16)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200580016849.7A CN1972884B (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| AT05744145T ATE553074T1 (en) | 2004-05-24 | 2005-05-24 | MOLECULARLY EMBOSSED, NITROSAMINE SELECTIVE POLYMERS AND METHOD FOR USE THEREOF |
| JP2007527126A JP5468736B2 (en) | 2004-05-24 | 2005-05-24 | Molecular imprinted polymer selective for nitrosamines and method of use thereof |
| PL05744145T PL1756023T3 (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| CA2565129A CA2565129C (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| MXPA06013628A MXPA06013628A (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same. |
| EA200602044A EA014824B1 (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymer selective to nitrosamines, method for manufacturing thereof (variants), kit, smoking article and a filter comprising polymer and methods of using polymer |
| BRPI0511416A BRPI0511416B8 (en) | 2004-05-24 | 2005-05-24 | molecular imprint polymer, kit, methods for preparing a molecular imprint polymer, to determine if tobacco product contains nitrosamines, to quantify the amount of nitrosamines in a tobacco product, to treat a tobacco product to reduce the level of a component target therein and to manufacture a tobacco material, smoking article, and smoke filter |
| AU2005244726A AU2005244726B2 (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| KR1020147023274A KR101556872B1 (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| KR1020067026477A KR101276988B1 (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| KR1020137005116A KR101505333B1 (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| ES05744145T ES2384260T3 (en) | 2004-05-24 | 2005-05-24 | Molecularly selective printed polymers for nitrosamines and methods for using them |
| EP05744145A EP1756023B1 (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| US11/604,003 US8807142B2 (en) | 2004-05-24 | 2006-11-21 | Molecularly imprinted polymers selective for nitrosamines and method of preparing the same |
| US14/327,509 US9844230B2 (en) | 2004-05-24 | 2014-07-09 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US57333704P | 2004-05-24 | 2004-05-24 | |
| US60/573,337 | 2004-05-24 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/604,003 Continuation US8807142B2 (en) | 2004-05-24 | 2006-11-21 | Molecularly imprinted polymers selective for nitrosamines and method of preparing the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2005112670A1 true WO2005112670A1 (en) | 2005-12-01 |
| WO2005112670A8 WO2005112670A8 (en) | 2006-08-24 |
Family
ID=35428207
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/SE2005/000773 WO2005112670A1 (en) | 2004-05-24 | 2005-05-24 | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
Country Status (16)
| Country | Link |
|---|---|
| US (2) | US8807142B2 (en) |
| EP (1) | EP1756023B1 (en) |
| JP (1) | JP5468736B2 (en) |
| KR (3) | KR101556872B1 (en) |
| CN (1) | CN1972884B (en) |
| AT (1) | ATE553074T1 (en) |
| AU (1) | AU2005244726B2 (en) |
| BR (1) | BRPI0511416B8 (en) |
| CA (1) | CA2565129C (en) |
| EA (1) | EA014824B1 (en) |
| ES (1) | ES2384260T3 (en) |
| MX (1) | MXPA06013628A (en) |
| PL (1) | PL1756023T3 (en) |
| UA (1) | UA88469C2 (en) |
| WO (1) | WO2005112670A1 (en) |
| ZA (1) | ZA200609378B (en) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008068153A2 (en) * | 2006-12-07 | 2008-06-12 | British American Tobacco (Investments) Limited | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
| US7709264B2 (en) * | 2006-09-21 | 2010-05-04 | Philip Morris Usa Inc. | Handheld microcantilever-based sensor for detecting tobacco-specific nitrosamines |
| JP2010522810A (en) * | 2007-03-27 | 2010-07-08 | ユニバーシティー オブ メリーランド,カレッジ パーク | Imprinted polymeric materials for binding to various targets such as viruses |
| WO2010085851A1 (en) * | 2009-01-29 | 2010-08-05 | Commonwealth Scientific And Industrial Research Organisation | Molecularly imprinted polymers |
| US8079369B2 (en) | 2008-05-21 | 2011-12-20 | R.J. Reynolds Tobacco Company | Method of forming a cigarette filter rod member |
| CN102423116A (en) * | 2011-11-07 | 2012-04-25 | 上海聚华科技股份有限公司 | Preparation method of pineapple tobacco flavor |
| EP2537427A1 (en) | 2008-05-21 | 2012-12-26 | R.J. Reynolds Tobacco Company | Cigarette filter having composite fiber structures |
| US20130139834A1 (en) * | 2006-12-07 | 2013-06-06 | British American Tobacco (Investments) Limited | Polymers Selective for Nitro-Containing Compounds and Methods of Using the Same |
| CN103776944A (en) * | 2014-01-13 | 2014-05-07 | 红云红河烟草(集团)有限责任公司 | Method for detecting NNAL and NNA in tobacco shred and cigarette smoke |
| US8807142B2 (en) | 2004-05-24 | 2014-08-19 | British American Tobacco (Investments) Limited | Molecularly imprinted polymers selective for nitrosamines and method of preparing the same |
| US8882647B2 (en) | 2005-09-23 | 2014-11-11 | R.J. Reynolds Tobacco Company | Equipment for insertion of objects into smoking articles |
| EP2965637A4 (en) * | 2013-06-19 | 2016-12-07 | Japan Tobacco Inc | PROCESS FOR PRODUCING TOBACCO RAW MATERIAL |
| CN111521716A (en) * | 2020-06-09 | 2020-08-11 | 甘肃烟草工业有限责任公司 | Cigarette raw material mixing uniformity evaluation method based on design value |
| US11166485B2 (en) | 2013-03-14 | 2021-11-09 | R.J. Reynolds Tobacco Company | Protein-enriched tobacco-derived composition |
Families Citing this family (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100846898B1 (en) | 2005-12-30 | 2008-07-17 | 엘지전자 주식회사 | Stand of video display device |
| JP5370712B2 (en) | 2008-02-21 | 2013-12-18 | 日東電工株式会社 | Acidic water-soluble target substance adsorbing polymer and method for producing the same |
| EP2138214A1 (en) * | 2008-06-27 | 2009-12-30 | British American Tobacco (Investments) Limited | A method for removing polycyclic aromatic hydrocarbons |
| FR2962549B1 (en) * | 2010-07-08 | 2012-08-24 | Commissariat Energie Atomique | DEVICE FOR ELECTRICAL DETECTION AND / OR QUANTIFICATION BY MOLECULAR FOOTPRINTING OF ORGANOPHOSPHORUS COMPOUNDS |
| US9192193B2 (en) | 2011-05-19 | 2015-11-24 | R.J. Reynolds Tobacco Company | Molecularly imprinted polymers for treating tobacco material and filtering smoke from smoking articles |
| US9474303B2 (en) | 2011-09-22 | 2016-10-25 | R.J. Reynolds Tobacco Company | Translucent smokeless tobacco product |
| US9420825B2 (en) | 2012-02-13 | 2016-08-23 | R.J. Reynolds Tobacco Company | Whitened tobacco composition |
| EP2864417B1 (en) * | 2012-06-21 | 2019-01-09 | Ligar Limited Partnership | Polymer and method of use |
| US9854841B2 (en) | 2012-10-08 | 2018-01-02 | Rai Strategic Holdings, Inc. | Electronic smoking article and associated method |
| US11412775B2 (en) | 2012-10-09 | 2022-08-16 | R.J. Reynolds Tobacco Company | Tobacco-derived composition |
| US10034988B2 (en) | 2012-11-28 | 2018-07-31 | Fontem Holdings I B.V. | Methods and devices for compound delivery |
| US8910640B2 (en) | 2013-01-30 | 2014-12-16 | R.J. Reynolds Tobacco Company | Wick suitable for use in an electronic smoking article |
| US10031183B2 (en) | 2013-03-07 | 2018-07-24 | Rai Strategic Holdings, Inc. | Spent cartridge detection method and system for an electronic smoking article |
| US20140261486A1 (en) | 2013-03-12 | 2014-09-18 | R.J. Reynolds Tobacco Company | Electronic smoking article having a vapor-enhancing apparatus and associated method |
| US20140261487A1 (en) | 2013-03-14 | 2014-09-18 | R. J. Reynolds Tobacco Company | Electronic smoking article with improved storage and transport of aerosol precursor compositions |
| US9661876B2 (en) * | 2013-03-14 | 2017-05-30 | R.J. Reynolds Tobacco Company | Sugar-enriched extract derived from tobacco |
| US9423152B2 (en) | 2013-03-15 | 2016-08-23 | R. J. Reynolds Tobacco Company | Heating control arrangement for an electronic smoking article and associated system and method |
| US9175052B2 (en) | 2013-05-17 | 2015-11-03 | R.J. Reynolds Tobacco Company | Tobacco-derived protein compositions |
| US20140356295A1 (en) | 2013-06-03 | 2014-12-04 | R.J. Reynolds Tobacco Company | Cosmetic compositions comprising tobacco seed-derived component |
| US9629391B2 (en) | 2013-08-08 | 2017-04-25 | R.J. Reynolds Tobacco Company | Tobacco-derived pyrolysis oil |
| US11503853B2 (en) | 2013-09-09 | 2022-11-22 | R.J. Reynolds Tobacco Company | Smokeless tobacco composition incorporating a botanical material |
| US10194693B2 (en) | 2013-09-20 | 2019-02-05 | Fontem Holdings 1 B.V. | Aerosol generating device |
| CN103601855B (en) * | 2013-09-30 | 2016-08-10 | 广东中烟工业有限责任公司 | A kind of molecular engram mono-dispersion microballoon that tobacco-specific nitrosamine is had specific adsorption function and preparation thereof and application |
| US10357054B2 (en) | 2013-10-16 | 2019-07-23 | R.J. Reynolds Tobacco Company | Smokeless tobacco pastille |
| US20150216237A1 (en) | 2014-01-22 | 2015-08-06 | E-Nicotine Technology, Inc. | Methods and devices for smoking urge relief |
| US9375033B2 (en) | 2014-02-14 | 2016-06-28 | R.J. Reynolds Tobacco Company | Tobacco-containing gel composition |
| DE102014008135A1 (en) * | 2014-06-06 | 2015-12-17 | Gfl Gesellschaft Für Lebensmittel-Forschung Mbh | Method for analytical authenticity testing of ingredients in food using artificial antibodies |
| CN105044252A (en) * | 2015-08-18 | 2015-11-11 | 云南中烟工业有限责任公司 | Pretreatment device for detection of nitrosamine marker and application thereof |
| US10869497B2 (en) | 2015-09-08 | 2020-12-22 | R.J. Reynolds Tobacco Company | High-pressure cold pasteurization of tobacco material |
| US10149844B2 (en) | 2015-09-16 | 2018-12-11 | Philip Morris Products S.A. | Inhalable nicotine formulations, and methods of making and using thereof |
| US9585835B1 (en) * | 2015-09-16 | 2017-03-07 | Sansa Corporation (Barbados) Inc. | Inhalable nicotine formulations and methods of making and using the same |
| US10532046B2 (en) | 2015-12-03 | 2020-01-14 | Niconovum Usa, Inc. | Multi-phase delivery compositions and products incorporating such compositions |
| US11612183B2 (en) | 2015-12-10 | 2023-03-28 | R.J. Reynolds Tobacco Company | Protein-enriched tobacco composition |
| US10757964B2 (en) | 2017-07-20 | 2020-09-01 | R.J. Reynolds Tobacco Company | Purification of tobacco-derived protein compositions |
| US12114688B2 (en) | 2017-10-24 | 2024-10-15 | Rai Strategic Holdings, Inc. | Method for formulating aerosol precursor for aerosol delivery device |
| CN107868164B (en) * | 2017-11-28 | 2020-04-24 | 南京大学 | Preparation method and application of nitrosamine disinfection byproduct molecularly imprinted polymer |
| US20190307082A1 (en) | 2018-04-05 | 2019-10-10 | R.J. Reynolds Tobacco Company | Oriental tobacco production methods |
| CN109007950B (en) * | 2018-05-25 | 2021-02-19 | 吉首大学 | Preparation method and application of N-nitrosamine poison composite imprinted sheet |
| CN113784634B (en) | 2019-03-08 | 2024-04-16 | 莱战略控股公司 | Lactic acid hydrolysis method for aerosol delivery devices |
| CN110128590A (en) * | 2019-05-15 | 2019-08-16 | 南通市产品质量监督检验所 | A kind of preparation method of the N- N-nitrosodimethylamine molecularly imprinted polymer using acrylamide as function monomer |
| CN112903831A (en) * | 2019-12-04 | 2021-06-04 | 中国科学院大连化学物理研究所 | Integrated sample processing system for metabolic glycoside derivatives in flue gas body fluid and application |
| US12102136B2 (en) * | 2020-07-08 | 2024-10-01 | Daniel Rush | Universal filtering mouthpiece for smoking devices |
| AU2021405529A1 (en) * | 2020-12-24 | 2023-08-10 | Ligar Limited Partnership | Method, system and/or apparatus for use of liquid or fluid carbon dioxide in extraction and/or solubilising source material and binding and/or elution with a molecularly imprinted polymer |
| US11491377B1 (en) | 2021-12-28 | 2022-11-08 | Acushnet Company | Golf club head having multi-layered striking face |
| US11850461B2 (en) | 2022-03-11 | 2023-12-26 | Acushnet Company | Golf club head having supported striking face |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994011403A1 (en) * | 1992-11-11 | 1994-05-26 | Klaus Mosbach | Artificial antibodies, method of producing the same and use thereof |
| US5587273A (en) * | 1993-01-21 | 1996-12-24 | Advanced Microbotics Corporation | Molecularly imprinted materials, method for their preparation and devices employing such materials |
| WO1999033768A1 (en) * | 1997-12-30 | 1999-07-08 | Klaus Mosbach | Materials for screening of combinatorial libraries |
| WO2000032648A1 (en) * | 1998-11-30 | 2000-06-08 | Dr. Gottschall Instruction | Method of producing a polymer network |
| US20030003587A1 (en) * | 2002-06-28 | 2003-01-02 | Murray George M | Molecularly imprinted polymer based sensors for the detection of narcotics |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4589428A (en) * | 1980-02-21 | 1986-05-20 | Philip Morris Incorporated | Tobacco treatment |
| SU1432067A1 (en) * | 1986-11-14 | 1988-10-23 | Башкирский государственный научно-исследовательский и проектный институт нефтяной промышленности | Method of producing polymeric flocculant |
| AT396862B (en) | 1991-10-22 | 1993-12-27 | Austria Tabakwerke Ag | METHOD FOR PRODUCING A CELLULOSE ACETATE CABLE AND CIGARETTE FILTER THEREOF |
| US5311886A (en) * | 1991-12-31 | 1994-05-17 | Imasco Limited | Tobacco extract treatment with insoluble adsorbent |
| RU2045921C1 (en) * | 1992-12-08 | 1995-10-20 | Товарищество с ограниченной ответственностью "Сфинкс" | Cigarette filter |
| US5810020A (en) * | 1993-09-07 | 1998-09-22 | Osmotek, Inc. | Process for removing nitrogen-containing anions and tobacco-specific nitrosamines from tobacco products |
| DE19541873A1 (en) * | 1995-11-09 | 1997-05-15 | Rhodia Ag Rhone Poulenc | Filter cigarette |
| RU2113810C1 (en) * | 1996-09-10 | 1998-06-27 | Институт биохимической физики РАН | Filter for cigarettes |
| US6082370A (en) * | 1998-02-09 | 2000-07-04 | Rousseau Research, Inc. | Cigarette with dry powered Vitamin E |
| WO2001065954A1 (en) | 2000-03-10 | 2001-09-13 | British American Tobacco (Investments) Limited | Tobacco treatment |
| KR100879193B1 (en) * | 2000-10-05 | 2009-01-16 | 니꼴라스 바스케비치 | Reduction of Nitrosamines in Tobacco and Tobacco Products |
| KR200322202Y1 (en) | 2003-02-06 | 2003-08-02 | 이형 | Extraction And Transparent Filter Cigarette |
| UA88469C2 (en) | 2004-05-24 | 2009-10-26 | Бритиш Американ Тобакко (Инвестментс) Лимитед | Molecularly imprinted polymer selective to nitrosamines, process for the preparation thereof (variants), using, methods for determining availability and qualitative content of nitrosamines in tobacco product, method for treating tobacco product, method for manufacturing tobacco product, smoking article and filter for tobacco smoke |
| GB0427901D0 (en) | 2004-12-21 | 2005-01-19 | Univ Cranfield | Virtual imprinting |
| GB201200878D0 (en) | 2012-01-19 | 2012-02-29 | British American Tobacco Co | Polymer compositions |
| TWI421037B (en) | 2006-12-07 | 2014-01-01 | British American Tobacco Co | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
| WO2008107271A1 (en) | 2007-03-05 | 2008-09-12 | Mip Technologies Ab | Imprinted polymers |
-
2005
- 2005-05-24 UA UAA200613295A patent/UA88469C2/en unknown
- 2005-05-24 AU AU2005244726A patent/AU2005244726B2/en not_active Ceased
- 2005-05-24 KR KR1020147023274A patent/KR101556872B1/en active IP Right Grant
- 2005-05-24 PL PL05744145T patent/PL1756023T3/en unknown
- 2005-05-24 CA CA2565129A patent/CA2565129C/en not_active Expired - Fee Related
- 2005-05-24 KR KR1020067026477A patent/KR101276988B1/en active IP Right Grant
- 2005-05-24 EP EP05744145A patent/EP1756023B1/en active Active
- 2005-05-24 EA EA200602044A patent/EA014824B1/en not_active IP Right Cessation
- 2005-05-24 ES ES05744145T patent/ES2384260T3/en active Active
- 2005-05-24 JP JP2007527126A patent/JP5468736B2/en not_active Expired - Fee Related
- 2005-05-24 BR BRPI0511416A patent/BRPI0511416B8/en not_active IP Right Cessation
- 2005-05-24 CN CN200580016849.7A patent/CN1972884B/en not_active Expired - Fee Related
- 2005-05-24 MX MXPA06013628A patent/MXPA06013628A/en active IP Right Grant
- 2005-05-24 WO PCT/SE2005/000773 patent/WO2005112670A1/en active Application Filing
- 2005-05-24 AT AT05744145T patent/ATE553074T1/en active
- 2005-05-24 KR KR1020137005116A patent/KR101505333B1/en active IP Right Grant
-
2006
- 2006-11-09 ZA ZA200609378A patent/ZA200609378B/en unknown
- 2006-11-21 US US11/604,003 patent/US8807142B2/en not_active Expired - Fee Related
-
2014
- 2014-07-09 US US14/327,509 patent/US9844230B2/en not_active Expired - Fee Related
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994011403A1 (en) * | 1992-11-11 | 1994-05-26 | Klaus Mosbach | Artificial antibodies, method of producing the same and use thereof |
| US5587273A (en) * | 1993-01-21 | 1996-12-24 | Advanced Microbotics Corporation | Molecularly imprinted materials, method for their preparation and devices employing such materials |
| WO1999033768A1 (en) * | 1997-12-30 | 1999-07-08 | Klaus Mosbach | Materials for screening of combinatorial libraries |
| WO2000032648A1 (en) * | 1998-11-30 | 2000-06-08 | Dr. Gottschall Instruction | Method of producing a polymer network |
| US20030003587A1 (en) * | 2002-06-28 | 2003-01-02 | Murray George M | Molecularly imprinted polymer based sensors for the detection of narcotics |
Non-Patent Citations (3)
| Title |
|---|
| A. ZANDER ET AL: "Analysis of Nicotine and its Oxidation Products in Nicotine Chewing Gum by a Molecularly Imprinted Solid-Phase Extraction.", ANALYTICAL CHEMISTRY., vol. 70, no. 15, 1 August 1998 (1998-08-01), pages 3304 - 3314, XP002995325 * |
| SELLERGREN B. ET AL.: "Highly enantioselective and substrate-selective polymers obtained by molecular imprinting utilizing noncovalent interactions. NMR and chromatographic studies on the nature of recognition", J.AM.CHEM.SOC., vol. 110, 1988, pages 5853 - 5860, XP002132606 * |
| Y. LIU ET AL: "Molecularly Imprinted Solid-Phase Extraction Sorbent for Removal of Nicotine from Tabacco Smoke", ANALYTICAL LETTERS., vol. 36, no. 8, 2003, pages 1631 - 1645, XP002995326 * |
Cited By (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8807142B2 (en) | 2004-05-24 | 2014-08-19 | British American Tobacco (Investments) Limited | Molecularly imprinted polymers selective for nitrosamines and method of preparing the same |
| US9844230B2 (en) | 2004-05-24 | 2017-12-19 | British American Tobacco (Investments) Ltd | Molecularly imprinted polymers selective for nitrosamines and methods of using the same |
| US11383477B2 (en) | 2005-09-23 | 2022-07-12 | R.J. Reynolds Tobacco Company | Equipment for insertion of objects into smoking articles |
| US10123562B2 (en) | 2005-09-23 | 2018-11-13 | R.J. Reynolds Tobacco Company | Equipment for insertion of objects into smoking articles |
| US9398777B2 (en) | 2005-09-23 | 2016-07-26 | R.J. Reynolds Tobacco Company | Equipment for insertion of objects into smoking articles |
| US9028385B2 (en) | 2005-09-23 | 2015-05-12 | R.J. Reynolds Tobacco Company | Equipment for insertion of objects into smoking articles |
| US8882647B2 (en) | 2005-09-23 | 2014-11-11 | R.J. Reynolds Tobacco Company | Equipment for insertion of objects into smoking articles |
| US7709264B2 (en) * | 2006-09-21 | 2010-05-04 | Philip Morris Usa Inc. | Handheld microcantilever-based sensor for detecting tobacco-specific nitrosamines |
| US9265283B2 (en) | 2006-12-07 | 2016-02-23 | British American Tobacco (Investments) Limited | Polymers selective for tobacco specific nitrosamines and methods of using the same |
| WO2008068153A2 (en) * | 2006-12-07 | 2008-06-12 | British American Tobacco (Investments) Limited | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
| JP2010511395A (en) * | 2006-12-07 | 2010-04-15 | ブリティッシュ アメリカン タバコ (インヴェストメンツ) リミテッド | Molecularly imprinted polymer having selectivity for tobacco-specific nitrosamines and method of use thereof |
| US20130139834A1 (en) * | 2006-12-07 | 2013-06-06 | British American Tobacco (Investments) Limited | Polymers Selective for Nitro-Containing Compounds and Methods of Using the Same |
| US9243096B2 (en) * | 2006-12-07 | 2016-01-26 | British American Tobacco (Investments) Limited | Polymers selective for nitro-containing compounds and methods of using the same |
| TWI421037B (en) * | 2006-12-07 | 2014-01-01 | British American Tobacco Co | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
| RU2504307C2 (en) * | 2006-12-07 | 2014-01-20 | Бритиш Америкэн Тобэкко (Инвестментс) Лимитед | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods for use thereof |
| WO2008068153A3 (en) * | 2006-12-07 | 2008-09-25 | British American Tobacco Co | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
| US8733369B2 (en) | 2006-12-07 | 2014-05-27 | British American Tobacco (Investments) Limited | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
| KR101426975B1 (en) * | 2006-12-07 | 2014-08-06 | 브리티쉬 아메리칸 토바코 (인베스트먼츠) 리미티드 | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
| US9169341B2 (en) | 2006-12-07 | 2015-10-27 | British American Tobacco (Investments) Limited | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using same |
| AU2007329060B2 (en) * | 2006-12-07 | 2011-05-19 | British American Tobacco (Investments) Limited | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
| US8889795B2 (en) | 2006-12-07 | 2014-11-18 | British American Tobacco (Investments) Limited | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using same |
| JP2010522810A (en) * | 2007-03-27 | 2010-07-08 | ユニバーシティー オブ メリーランド,カレッジ パーク | Imprinted polymeric materials for binding to various targets such as viruses |
| US8079369B2 (en) | 2008-05-21 | 2011-12-20 | R.J. Reynolds Tobacco Company | Method of forming a cigarette filter rod member |
| EP2537427A1 (en) | 2008-05-21 | 2012-12-26 | R.J. Reynolds Tobacco Company | Cigarette filter having composite fiber structures |
| WO2010085851A1 (en) * | 2009-01-29 | 2010-08-05 | Commonwealth Scientific And Industrial Research Organisation | Molecularly imprinted polymers |
| CN102423116A (en) * | 2011-11-07 | 2012-04-25 | 上海聚华科技股份有限公司 | Preparation method of pineapple tobacco flavor |
| CN102423116B (en) * | 2011-11-07 | 2013-03-20 | 上海聚华科技股份有限公司 | Preparation method for pineapple spice for cigarette |
| WO2013108001A1 (en) | 2012-01-19 | 2013-07-25 | British American Tobacco (Investments) Limited | Selective separation of nitroso-containing compounds |
| US11166485B2 (en) | 2013-03-14 | 2021-11-09 | R.J. Reynolds Tobacco Company | Protein-enriched tobacco-derived composition |
| US11375741B2 (en) | 2013-03-14 | 2022-07-05 | R.J. Reynolds Tobacco Company | Protein-enriched tobacco-derived composition |
| RU2633205C2 (en) * | 2013-06-19 | 2017-10-11 | Джапан Тобакко Инк. | Method of producing raw tobacco material |
| EP2965637A4 (en) * | 2013-06-19 | 2016-12-07 | Japan Tobacco Inc | PROCESS FOR PRODUCING TOBACCO RAW MATERIAL |
| CN103776944A (en) * | 2014-01-13 | 2014-05-07 | 红云红河烟草(集团)有限责任公司 | Method for detecting NNAL and NNA in tobacco shred and cigarette smoke |
| CN111521716A (en) * | 2020-06-09 | 2020-08-11 | 甘肃烟草工业有限责任公司 | Cigarette raw material mixing uniformity evaluation method based on design value |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE553074T1 (en) | 2012-04-15 |
| PL1756023T3 (en) | 2012-09-28 |
| BRPI0511416B1 (en) | 2016-06-21 |
| EA200602044A1 (en) | 2007-06-29 |
| CN1972884A (en) | 2007-05-30 |
| CA2565129C (en) | 2012-12-11 |
| EA014824B1 (en) | 2011-02-28 |
| WO2005112670A8 (en) | 2006-08-24 |
| KR20070033356A (en) | 2007-03-26 |
| EP1756023B1 (en) | 2012-04-11 |
| UA88469C2 (en) | 2009-10-26 |
| BRPI0511416B8 (en) | 2016-08-02 |
| US20070186940A1 (en) | 2007-08-16 |
| MXPA06013628A (en) | 2007-03-23 |
| US9844230B2 (en) | 2017-12-19 |
| ES2384260T3 (en) | 2012-07-03 |
| US20140332015A1 (en) | 2014-11-13 |
| KR101556872B1 (en) | 2015-10-01 |
| KR101276988B1 (en) | 2013-06-24 |
| KR20130036360A (en) | 2013-04-11 |
| CN1972884B (en) | 2014-03-26 |
| EP1756023A1 (en) | 2007-02-28 |
| JP2008500442A (en) | 2008-01-10 |
| ZA200609378B (en) | 2009-02-25 |
| US8807142B2 (en) | 2014-08-19 |
| CA2565129A1 (en) | 2005-12-01 |
| AU2005244726A1 (en) | 2005-12-01 |
| AU2005244726B2 (en) | 2010-08-26 |
| JP5468736B2 (en) | 2014-04-09 |
| KR101505333B1 (en) | 2015-03-23 |
| KR20140108600A (en) | 2014-09-11 |
| BRPI0511416A (en) | 2007-12-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1756023B1 (en) | Molecularly imprinted polymers selective for nitrosamines and methods of using the same | |
| EP2094119B1 (en) | Molecularly imprinted polymers selective for tobacco specific nitrosamines and methods of using the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KM KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NG NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DPEN | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2005244726 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2565129 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200609378 Country of ref document: ZA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007527126 Country of ref document: JP |
|
| ENP | Entry into the national phase |
Ref document number: 2005244726 Country of ref document: AU Date of ref document: 20050524 Kind code of ref document: A |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005244726 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11604003 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/2006/013628 Country of ref document: MX Ref document number: 200580016849.7 Country of ref document: CN Ref document number: 1200601942 Country of ref document: VN |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020067026477 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005744145 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200602044 Country of ref document: EA |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005744145 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020067026477 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 11604003 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: PI0511416 Country of ref document: BR |