Silver iodide

| |
| |
| Names | |
|---|---|
| IUPAC name
Silver(I) iodide
| |
| Other names
Argentous iodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.125 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AgI | |
| Molar mass | 234.77 g/mol |
| Appearance | yellow, crystalline solid |
| Odor | odorless |
| Density | 5.68 g/cm3, solid[1] |
| Melting point | 558 °C (1,036 °F; 831 K)[1] |
| Boiling point | 1,506 °C (2,743 °F; 1,779 K)[1] |
| 0.03 mg/L (20 °C)[1] | |
Solubility product (Ksp)
|
8.52 × 10 −17[2] |
| −80.0·10−6 cm3/mol[3] | |
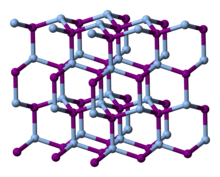
| Structure[5] | |
| Hexagonal, hP4 | |
| P63mc, No. 186 | |
a = 0.4591 nm, c = 0.7508 nm α = 90°, β = 90°, γ = 120°
| |
Formula units (Z)
|
2 |
| 4.55 D[4] | |
| Thermochemistry[6] | |
Heat capacity (C)
|
56.8 J·mol−1·K−1 |
Std molar
entropy (S⦵298) |
115.5 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−61.8 kJ·mol−1 |
Gibbs free energy (ΔfG⦵)
|
−66.2 kJ·mol−1 |
| Hazards | |
| GHS labelling:[7] | |

| |
| Warning | |
| H410 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | Sigma-Aldrich |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Silver iodide is an inorganic compound with the formula AgI. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a grey colouration. The silver contamination arises because some samples of AgI can be highly photosensitive. This property is exploited in silver-based photography. Silver iodide is also used as an antiseptic and in cloud seeding.
Structure
[edit]The structure adopted by silver iodide is temperature dependent:[8]
- Below 420 K, the β phase of AgI, with the wurtzite structure, is most stable. This phase is encountered in nature as the mineral iodargyrite.
- Above 420 K, the α phase becomes more stable. This motif is a body-centered cubic structure which has the silver centers distributed randomly between 6 octahedral, 12 tetrahedral and 24 trigonal sites.[9] At this temperature, Ag+ ions can move rapidly through the solid, allowing fast ion conduction. The transition between the β and α forms represents the melting of the silver (cation) sublattice. The entropy of fusion for α-AgI is approximately half that for sodium chloride (a typical ionic solid). This can be rationalized by considering the AgI crystalline lattice to have already "partly melted" in the transition between α and β polymorphs.
- A metastable γ phase also exists below 420 K with the zinc blende structure.

Preparation and properties
[edit]Silver iodide is prepared by reaction of an iodide solution (e.g., potassium iodide) with a solution of silver ions (e.g., silver nitrate). A yellowish solid quickly precipitates. The solid is a mixture of the two principal phases. Dissolution of the AgI in hydroiodic acid, followed by dilution with water, precipitates β-AgI. Alternatively, dissolution of AgI in a solution of concentrated silver nitrate followed by dilution affords α-AgI.[10] Unless the preparation is conducted in dark conditions, the solid darkens rapidly, the light causing the reduction of ionic silver to metallic. The photosensitivity varies with sample purity.
Cloud seeding
[edit]
The crystalline structure of β-AgI is similar to that of ice, allowing it to induce freezing by the process known as heterogeneous nucleation. Approximately 50,000 kg are used for cloud seeding annually, each seeding experiment consuming 10–50 grams.[11] (see also Project Stormfury, Operation Popeye).[citation needed]
Safety
[edit]Extreme exposure can lead to argyria, characterized by localized discolouration of body tissue.[12]
References
[edit]- ^ a b c d Haynes, p. 4.84
- ^ Haynes, p. 5.178
- ^ Haynes, p. 4.130
- ^ Haynes, p. 9.65
- ^ Yoshiasa, A.; Koto, K.; Kanamaru, F.; Emura, S.; Horiuchi, H. (1987). "Anharmonic thermal vibrations in wurtzite-type AgI". Acta Crystallographica Section B: Structural Science. 43 (5): 434–440. Bibcode:1987AcCrB..43..434Y. doi:10.1107/S0108768187097532.
- ^ Haynes, p. 5.35
- ^ "C&L Inventory". echa.europa.eu. Retrieved 15 December 2021.
- ^ Binner, J. G. P.; Dimitrakis, G.; Price, D. M.; Reading, M.; Vaidhyanathan, B. (2006). "Hysteresis in the β–α Phase Transition in Silver Iodine" (PDF). Journal of Thermal Analysis and Calorimetry. 84 (2): 409–412. CiteSeerX 10.1.1.368.2816. doi:10.1007/s10973-005-7154-1. S2CID 14573346.
- ^ Hull, Stephen (2007). "Superionics: crystal structures and conduction processes". Rep. Prog. Phys. 67 (7): 1233–1314. doi:10.1088/0034-4885/67/7/R05. S2CID 250874771.
- ^ O. Glemser, H. Saur "Silver Iodide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1036-7.
- ^ Phyllis A. Lyday "Iodine and Iodine Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a14_381
- ^ "Silver Iodide". TOXNET: Toxicogy Data Network. U.S. National Library of Medicine. Retrieved 9 March 2016.
Cited sources
[edit]- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
