Abstract
In recent times, there has been rapid progress and achievement in the development of nanoparticle production in a microfluidic environment. Microfluidics technology harnesses the fluid mechanics to generate nanoparticles with a unique size and finely controllable shape that can be used for various applications like drug delivery, biological sciences, healthcare, and food industries. The nanoparticles are generally distinguished from fine particles to coarse particles due to their smaller size and unique material properties like chemical, physical, biological, and optical. However, the conventional methods require bulky instruments, expensive autoclaves, consume more power, high thermal loss, and require more time for the synthesis. Further, it is very challenging to automate, integrate, and miniaturize the conventional device on a single platform for synthesizing micro-and nanoscale particles. There has been considerable advancement in the development of microfluidic devices in the last few years for nanoparticle synthesis. The microfluidic device unveils several features such as portability, transparency in operation, controllability, and stability with a marginal reaction volume. The microfluidic-based nanoparticle synthesis also allows rapid processing and increased efficiency of the technique by using minimum peripherals for its operation. In this review article, we have discussed the microfluidic devices that are used for synthesizing various nanoparticles for different applications. This review summarizes the value-chain to develop microfluidic devices, including designs, fabrication techniques, and other related methodologies, to create nanoparticles in a controlled and selective manner.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Microfluidic based devices are developed to manipulate the fluid flow in the microchips by significantly minimizing the volume of reagents and to manifest inherently by effective and efficient transfer of mass and heat due to their large surface area to volume ratio [1]. Effectual blending and rapid mixing at microliter to picoscale volume make the microfluidic systems more suitable for synthesizing nanoparticles in a controllable and selective manner with sizes, shapes, morphology, and their unique properties [2]. The synthesis of nanoparticles requires controlled functioning and also needs coordinated processing for better outcome of quality properties [3]. The miniaturization of the nanoparticle synthesis process offers several prospects for nanomaterial production and further enables a wide application in biopharmaceutical [4], biological [5], biomedicinal [6], and biochemical domains [7]. The utilization of microfluidic devices for nanomaterial synthesis is useful due to numerous features, comprising high throughput, flexibility, transparency, efficiency, reproductively, and precise, for designing a multi-pace strategy [2, 3]. The microfluidic devices provide several benefits such as smaller in size, low-cost, minimal sample reagents with safe operation, and user-friendly environment [8]. Microfluidics devices can generate homogeneous blends particles in a much controllable and intended manner [9]. Now-a-days researchers have relied on the miniaturized microfluidic platform to further synthesize nanoparticles to achieve higher quality optical, physical, biological, and chemical properties for diverse applications [10]. Recently, for nanomaterial synthesis, microfluidic systems have been designed to regulate and coordinate the required properties with desirable characteristics comprise composite materials, double shells, and functional surfaces [11].
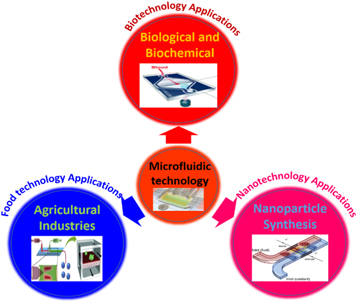
Microfluidics is the part of science and technology encompassing the design and development of miniaturized devices that control, coordinates, or manipulate micro-volumes of fluids, through the microchannels with dimensions ranging from tens to hundreds of micrometers [12]. This emerging technology has been widely utilized in a variety of areas like molecular biology, microelectronics, and nanotechnology. In microfluidic devices, the processing reaction sample reservoir is often called a microfluidic reactor or container [13]. The microfluidic reactor, a measure of a vast area of microscale fabrication, deals with a minimal volume of a streamlined fluid [14]. Even though the microfluidic reactors were initially developed in the early 1995s, cutting-edge technology fastened the process of development in recent years which includes design, process, utilization, and fabrication of microfluidic systems [15]. This was due to several distinct applications in biotechnology [16], nanotechnology [17], pharmaceutical [18], and advanced chemical synthesis [19]. It also finds applications in chemical industries like a reasonable synthesis of chemicals, crystallization, diagnosis, and combinatorial synthesis [20]. Figure 1 shows the microfluidic technology utilized in various applications. Microfluidic reactors provide several potential perks in the chemical industry because of its superior control on over-concentration, safer working environment, and high-throughput [21]. Thus, it is clear that these advancements in microfluidic systems, linked with nanotechnology, are outset to overlay the path to harness the increasing number of benefits in the future [22].
Figure 1. Microfluidic platform used for various applications.
Download figure:
Standard image High-resolution imageMicrofluidics is characterized by laminar based fluid flow at the microscale dimension, and markedly improved heat/mass transfer [23]. In addition, together with the benefits of flexible manipulation and large-scale integration, microfluidic technology has been hastily developed as one of the most significant platforms in the field of purposeful biomaterial synthesis [24]. Compared to biomaterials aided by conventional approaches, functional biomaterials produced by microfluidics are with superior performances and properties, because of their controllable composition and morphology, which have shown great benefits and potential in the field of tissue engineering, biosensing, bioimaging, and biomedicine [25, 26].
A nanoparticle is usually defined as a particle of matter that typically occurs in the range of nanometers (nm) in diameter and is composed of carbon, organic matter, metal oxides, or metal [27]. Nanoparticles are generally distinguished from fine particles to coarse particles due to their smaller size and unique material properties [28]. These properties include quantum mechanical effects, large surface to volume ratio, increased reactivity or stability that offers an extensive range of benefits over regular materials in fields such as agriculture, medicine, electronics, and engineering [29–32].
Further, in the nano-scale, the optical, physical, chemical, and biological properties are strongly influenced by the particle dimension size and pattern morphologies. Nanoparticle synthesis refers to methods for producing nanoparticles, which occur extensively in nature and are entities of study in several sciences such as biology, physics, geology, and chemistry [33, 34].
The generation of nanoparticles with explicit properties is a significant branch of nanotechnology. The synthesis of the nanoparticle can be classified into two categories, top-down and bottom-up. Nanoparticle properties are meticulously associated with their size, composition, shape, surface structure, morphology [35, 36]. The fundamental of nanoparticle synthesis parameters comprises blending, reaction time, temperature, and reagent concentration. The aforementioned parameters play a substantial role in synthesizing nanoparticles effectively and efficiently [37, 38].
In this review article, various methods and approaches involved in the synthesis of nanoparticles both in conventional and microfluidic approaches have been discussed. Further, different applications of nanoparticle synthesis have also been deliberated. Specific emphasis is given on the present trend in synthesizing nanoparticles using microfluidic technology to comprehend real-time and miniaturized processes with automation, fabrication, and integration. The Point-of-Care (POC) testing is a platform that permits patient diagnoses in the physician's office, an ambulance, the field, the home, or in the hospital [39]. Here, the analysis is performed where healthcare is provided close to or near the forbearing. The outcomes of care are timely, and let rapid treatment to the patients. Meanwhile, this can be further applied for point-of-care applications, mainly in synthesizing nanoparticles for numerous applications [40]. In this exertion, the design and advancement of recent devices on the miniaturized microfluidic platform have been discussed. This includes several parameters like the designing of microchannel, material and fabrication method, and characterization of particles. Finally, the potential, challenges, and future prospects associated with microfluidic devices for nanoparticle synthesis have been discussed.
1.1. Categorization of nanoparticles
Generally, the nanomaterials are categorized into three groups mainly, organic, inorganic, and carbon-based particles.
1.1.1. Organic nanoparticles
The organic nanomaterials are generally non-toxic, biodegradable and few materials in particular with micelles and liposomes has a cavity core usually called nanocapsules, and are very sensitive to electromagnetic radiation and thermal gradients like light and heat. Liposomes, dendrimers, ferritin, and micelles are commonly considered organic nanoparticles. The organic nanoparticles have peculiar characteristics that drive them to the ultimate option for delivery of drug [41]. These are mostly used in biomedical and bio-pharmacy fields in the delivery of drug schemes as they are effectual and further it can be easily infused on an explicit area of the body often called targeted drug delivery [42].
1.1.2. Inorganic nanoparticles
The inorganic nanomaterials generally comply with metal and metal oxide. Metal-based nanomaterials are synthesized to nanometric dimensions from metals by either constructive or destructive approaches. The most common metals utilized for synthesizing nanoparticle are iron (Fe), manganese (Mn), zinc (Zn), nickel (Ni), iron (Fe), gold (Au), aluminium (Al), silver (Ag), and copper (Cu). These nanoparticles have surface characteristics like pore size, large surface to volume ratio, shapes (cylindrical, spherical, color), crystalline structure, amorphous structure, and surface charge density [43]. Also, these nanoparticles are sensitive to environmental factors like sunlight, moisture, air, and heat. The metal oxide-based nanoparticles are synthesized to transform the properties of their corresponding metal-based particles [44]. Generally, herein, the titanium (Ti), iron (Fe), nickel (Ni), zinc (Zn) oxides are utilized, and synthesized typically are not the pure form. For instance, nanomaterials of nickel (Ni) instantaneously oxidizes to nickel oxide (NiO2) in the existence of oxygen at ambient temperature that mainly upsurges its reactivity associated with nickel nanomaterials. These nanomaterials have better efficiency and increased reactivity. The Lanthanide series transition metals are often collectively known as the rare earth elements. comprises the 15 metallic chemical elements, from lanthanum through the lutetium [45]. All but one of the lanthanides are f-block elements, corresponding to the substantial 4 f electron shell; reliant on the source, either lanthanum or lutetium is contemplated as a d-block element, but is included because of its chemical likenesses with the other 14 elements. Further, they are called lanthanides because the elements in the series are chemically similar to lanthanum. Lanthanides superficially resemble the 3 d transition metals, for instance, in forming colored compounds, but there are significant variances [46]. Their reactivity is greater, resembling the Group II elements such as magnesium, reflecting the large negative reduction potentials of the ions.
1.1.3. Carbon-based nanoparticles
The carbon-based nanoparticles are usually made up of complete carbon particles. Some of the popular nanoparticles include graphene, carbon black, carbon nanotubes (CNT), fullerenes, and carbon nanofibers [47]. Graphene oxide and few-layered graphene are also categorized under carbon-based nanoparticles. These kinds of nanoparticles are new and applicable in other various applications, mostly the few-layered graphene nanoparticles.
For many years, classical synthetic chemistry has produced and synthesized micro/nanoparticles wherein most methods, apparatus, and equipment have continued to be the same without much changes in the appearance of the traditional device. The classical approaches for synthesis of nanoparticle include nucleation followed by emulsion [48], growth [49], precipitation [50], grinding [51], and decomposition [52]. Most procedures in this approach work are on bench-top batch processes and are generally developed for bulk production of nanoparticles. But these existing methods typically have severe restrictions in the controlled production of monodispersed and homogenous nanoparticles and these normally create an extensive distribution of size [53]. The capability to rapidly and controllably that create a homogenous reactant mixture at the beginning of a reaction is desirable. Indeed, the effect of mixing on the extent of a reaction and product circulation is vital in microreactor design [54]. For biological and biomedical applications, the dominance of nanoparticle requirements has to lead to the necessity of their enormous quantities, the standards for the production of nanoparticle synthesis devices, and their approaches. Presently, researchers have been seeking alternative ways for synthesizing nanoparticles to improve and overwhelm the existing challenges associated with classical approaches built on nanotechnology's propitious capabilities [55].
1.1.4. Polymeric nanoparticles
Polymeric nanomaterials are extensively used as carriers in the pharmaceutical industry for controlled and sustained release in drug delivery systems. Polymeric nanoparticles can be either nanocapsules or nanosphere [56]. A couple of tactics utilized for the groundwork of polymeric nanomaterials are the top-down approach and the bottom-up approach. In the top-down approach, a scattering of preformed polymers generates polymeric nanomaterials, whereas, on the other hand, the bottom-up approach polymerization of monomers leads to the construction of polymeric nanomaterials [57]. The most extensively used synthetic polymers are polylactide–polyglycolide, polylactide, copolymers, polycaprolactones, and polyacrylates [58]. Among the several natural polymers, albumin, alginate, or chitosan have been broadly explored [59]. Numerous methods are used for the preparation of polymeric nanomaterials, namely, solvent diffusion, solvent evaporation, polymerization, and spontaneous emulsification.
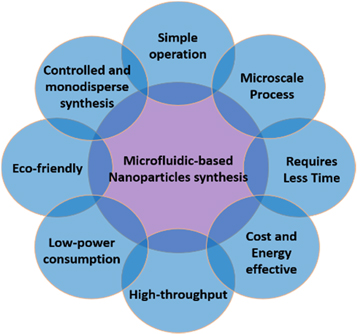
In comparison with conventional systems utilized for several decades, the microfluidic devices offer widespread benefits such as homogenous reactions, high sensitivity, inexpensive, low power consumption, shorter process time, high-throughput, and highly integrated multifunction. Besides, the scaling effect leads to distinctly new phenomena, allowing novel applications such as a high degree of concurrent execution and high-speed serial processing. The difficulties in conventional batch processes include large-scale conveyance and storage which also includes safety and health factors, such as seepage and eruption of toxic and combustible solvents, which are minimalized and mostly negligible in microfluidic devices. Figure 2 illustrates various advantages of using microfluidic devices for nanoparticles synthesis.
Figure 2. Schematic representation of various advantages using microfluidic devices for nanoparticles synthesis.
Download figure:
Standard image High-resolution image1.2. Nanomaterial properties
The properties of nanomaterials are usually classified into two types chemical and physical.
1.2.1. Chemical properties
The chemical reactivity of nanomaterials with stability, sensitivity, and target influencing parameters, like heat, air, background, moisture, sunlight, and atmosphere, delineate its applications. The anti-fungoid, toxicity and disinfection, properties of nanomaterials are extremely crucial for the environment and biological applications. Eroding [60], oxidation [61], flammability, anti-corrosive, and diminution features of the nanomaterials describe their corresponding applications. In chemical properties, chemical reactivity refers to the rate at which a chemical element tends to undergo a chemical reaction at the desired time. In pure compounds, reactivity is regulated by the chemical properties of the sample. For instance, grinding a sample to a higher specific superficial area enhances its reactivity. In impure compounds, the reactivity is also pretentious by the presence of contaminants. Whereas, in crystalline compounds, the crystalline form can also affect reactivity [62]. However, in all cases, reactivity is mainly due to the sub-atomic properties of the compound. The chemical reactivity of a substance covers its behavior in which it decomposes, forms new substances by addition of atoms from another reactant or reactants, interacts with two or more other reactants to form two or more products [63].
1.2.2. Physical properties
The physical properties consist of optical features like the color of the nanomaterial, its bright beam penetration, reflection, refraction and absorption abilities, and ultraviolet (UV) reflection and absorption capabilities in a reaction or even when treated onto a layer. It also comprises properties of mechanical variables like tensile, elastic, strengths, ductile, and flexibility that plays an important role in their application [64]. Electrical and magnetic properties, such as resistivity, conductivity, and conductivity, have led a way for nanomaterials to be utilized in advanced electronics and conductivity of temperature range in renewable energy applications. Other properties like hydrophobicity, suspension, hydrophilicity, settling, dispersion appearances have originated their path in the current trend of technology [65].
1.3. Diverse approaches and methods of nanoparticles synthesis
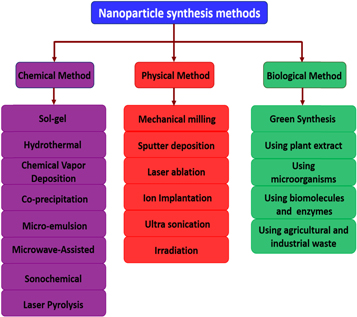
The nanoparticles synthesis process can be classified into two main approaches as bottom-up and top-down approaches. Further in general, the nanoparticle synthesis methods can be separated into three groups: physical, biological, chemical ways, and means [66, 67]. Figure 3 represents top-down and bottom-up approaches for nanomaterial production.
Figure 3. Top-down and bottom-up approaches for synthesizing nanoparticles.
Download figure:
Standard image High-resolution image1.3.1. Bottom-up method
The bottom-up or a construction system is the accumulation of material from smaller molecules to certain clusters of nanomaterials. This method contains assembling or coalescence of molecules to produce a diverse range of nanoparticles. The most frequently utilized bottom-up approaches for the generation of nanoparticles are hydrothermal, sol-gel, pyrolysis, biosynthesis, chemical vapor deposition (CVD), and spinning. Among these, the hydrothermal method is the most admired and garnering attention in synthesizing nanoparticles.
Bottom-up approaches depend on the molecular self-assembly phenomenon associated with chemical and physical interactions at the nanoscale which assemble initial building blocks into macroscopic structures [68]. The molecular interactivity comprises noncovalent bonds, such as ionic and hydrogen bonds. As element size decreases in nanofabrication, bottom-up approaches offer a progressively significant complement to top-down techniques [69]. Creativeness for bottom-up approaches comes from biological schemes, where nature has harnessed chemical forces to produce essentially all the assemblies needed by life [70]. This approach infers understanding the building blocks and then assembling them into a beneficial structure that can be used for various applications such as biological, clinical, and biochemical [71]. For nanoparticle and multilayer amalgams used in assertive loading environments, this needs an understanding of the length scales coordinating the toughness and strength of the blocks [72].
1.3.1.1. Sol-gel
The sol-gel is categorized under the bottom-up approach utilized to synthesis nanomaterial. Herein, this method is a colloidal concentration of solid deferred in a fluid juncture and gel is a solid macroparticle immersed in a reaction. The sol-gel method is one of the most preferred schemes for synthesizing nanoparticles because of its simple operation process. Generally, this process describes a wet-chemical process comprising a biochemical reaction performing as a precursor for a unified system of different materials. Typically, the precursor in the sol-gel process is formed by metal oxides and chlorides. The chemical compound is later diffused in a sample fluid utilizing sonication, stirring, or shaking. The subsequent reaction sample comprises both solid and fluidic phases. In the later stage, the obtained solution is separated and the purified solution is recovered by filtration, sedimentation, centrifugation, and the wetness is further eliminated by drying it in the micro oven.
1.3.1.2. Spinning
The synthesizing of the nanoparticle by the spinning method is supported by a spinning disc reactor (SDR). It comprises a rotary platter with an internal cavity or a container where the corporeal variables like thermal gradients can be coordinated and monitored. Often the container is occupied with nitrogen. hydrogen or any other idle gases to eradicate oxygen present in the interior chamber and evade chemical concentrations. The rotation disc is operated at various speeds where the fluidics such as water and precursor are pumped inside the chamber. Due to the spinning process, the molecules get fused, and further, it is precipitated, dried, and collected.
1.3.1.3. Chemical vapour deposition (CVD)
The chemical vapor deposition method is utilized to deposit particles on a thin film of gaseous reagents onto a particle substrate. The reaction chamber is the medium for carrying out deposition at ambient thermal grading by merging gas atoms. A chemical concentration happens when a warm substance emanates in touch with unified gas. This concentration process generates a thin film product on the superficial material which is improved and utilized. The CVD has advantages like a uniform, highly pure, strong, and hard nanoparticles. The shortcoming of the CVD process is the necessity of specific equipment and gaseous products which can be highly toxic.
1.3.1.4. Pyrolysis
Pyrolysis is the most commonly utilized approach in nanoparticle synthesis mainly used in industries for a very large-scale manufacturing process. It encompasses burning a chemical compound with a flame at a high steam rate. The precursor can be either fluidic or vapor which is transferred into the oven at a very high rate of pressure via a tiny hole where flame can be applied. The gases or combustion is later air categorized to recuperate the nanoparticles. Few furnaces use a laser beam or plasma method, instead of utilizing flame, which is used to generate high thermal output. The benefits of pyrolysis are easy to operate, low-cost, effective, and efficient processes with great outcomes.
1.3.1.5. Biosynthesis
The biosynthesis method, sometimes also called green synthesis and an environment-friendly strategy for nanoparticle synthesis. This method is biodegradable and non-toxic. Biosynthesis makes use of plant extracts, bacteria, bacillus, fungi besides the chemical compounds to generate nanomaterial as a replacement for classical chemicals for capping purposes and bioreduction. Because of its unique approach and properties, it is widely used in biological and biomedicinal applications.
1.3.2. Top-down method
Top-down or a destructive technique is the diminution of a mass particle to a nanometric measure of nanoparticles. Here the synthesis is initialized through a bulk counterpart that leaches out systematically process-by-process resulting in the production of fine nanoparticles. The top-down approach consists of different methods that are widely used in synthesizing nanoparticles for mass production such as nanolithography, electron beam lithography, mechanical milling, sputtering, thermal decomposition, and sputtering.
1.3.2.1. Mechanical milling
The mechanical milling method is one of the widely utilized to generate several nanomaterials. The mechanical grinding is utilized for refining and post-tempering of nanomaterials throughout the production wherein the various components are milled in an idle environment. The manipulating aspects in mechanical milling are plastic deformity which drives to a nanoparticle with varied shapes, fracture results in reduced nanoparticle appearance, and cold bonding results in enhanced nanoparticle dimension.
1.3.2.2. Nanolithography
Nanolithography is a survey of producing nanometric scales with a framework of minimum aspect ranging from 1 nm to 100 nm. Many nanolithographic processes are available for synthesizing nanoparticles for example scanning probe lithography, electron-beam, nanoimprint, optical, and multiphoton. The major benefit of using this method is to create a cluster from a solo nanomaterial with a specified size and shape. The only drawback of this method is that the necessity of complex and costlier bulky instruments.
1.3.2.3. Laser ablation
The laser ablation synthesis in solution (LASiS) is a process for the synthesis of nanoparticles created from different solutions. The irradiation of metal immersed in a fluidic phase by a laser light shrinks a plasma plume that creates small clusters of nanomaterials. It is a consistent means under a top-down approach that offers an alternative reaction to the classical chemical diminution of metals to produce them on a laser light source for nanoparticles. The LASiS offer a stable generation of nanomaterials in organic solutions.
1.3.2.4. Sputtering
Sputtering is a method that incorporates the supplanting of nanomaterials on a superficial layer by expelling materials from it by striking with ions. Further, the sputtering method is frequently used for the deposition of a thin film of nanomaterials trailed by a tempering process. The layer of the film, thermal range, and reaction time of substrate form and annealing describes the size and shape of nanomaterials.
1.3.2.5. Thermal decomposition
The thermal decomposition method is endothermic biochemical corrosion usually generated by warming up the interrupts involved in the bonding of precursor. At the desired thermal range of elements will get chemically decomposed undergoing a chemical reaction synthesizing nanoparticles.
2. Materials and methods
The microreactors are generally defined as miniaturized solvent-based micro-devices which are made-up of precision engineering methods and standards. The prerequisite for manufacturing the microreactors and microchannel for synthesizing nanoparticles in a microfluidic environment at a smaller scale depends on the area of application and relies on the resources and fabrication techniques. The microfabrication techniques to create a microchannel or a microreactor, generally, can be categorized into two kinds, direct and indirect methods. In the direct approach of microfabrication, a single microreactor device is designed and fabricated at a time in a substrate. In the indirect approach, the patterns are produced on mold or master which can be utilized many times to make the replicas.
The selection of materials in nanoparticle synthesis is an important task based on the field of application which has expanded with the growth of miniaturized microfluidic devices. A wide diversity of materials with a broad range of mechanical, optical, electrical, chemical properties can be utilized for producing microreactors. Typically, the material substrate used in the development of microfluidic devices are glass, silicon, metals, and polymers. Various microfabrication methods are applied depending on the material used in the process such as photolithography [73], soft lithography [74], embossing [75], molding [76], laser ablation [77].
The general properties of different resources can be used for the fabrication of microchannel or microreactor in the development of microfluidic devices. Microfluidic technology shares various similar principles to the fundamental challenges in inkjet technologies and to regulate supramolecular gels, mainly the requirement is to comprehend the surface tension of the fluid and rheology as well as the role of the microfluidic channel micro and nanostructures [78]. With the extensive range of biological samples such as plasma, saliva, blood, and urine the variance in fluid properties leads to a very clear requirement for robust and repeatable systems [79]. To achieve this for a wider range of applications and to certify a clear pathway to manufacture, microfluidics technology are capable of tackling simultaneously the challenges encountered in delivering measurable diagnostics and the manufacturability of the engineering solutions [80]. Manufacturing of microfluidic systems requires simple, traceable, techniques that can be rendered to both a pertinent scale and meet the appropriate manufacturing standards for biomedical devices [81].
Scale-up properties are the challenging procedure in nanoparticle synthesis that includes the construction of nanostructures for greater or commercial manufacture. The parameters affecting the scaling-up process are a reduced abundance of raw material, difficulty in manipulation of concentration and composition of molecules. Formulating such nanosystems can be tedious and pose several challenges at both the preclinical and clinical phases of drug development [82] using conventional scale-up processes. The main drawback occurs when transitioning from preclinical formulations to scaling-up generation for clinical trials [83]. This needs a large amount of resources and time in this process. In the microfluidic environment the nanoparticle synthesis, strategies have been developed to offer a successful approach to scale-up the nanomaterial synthesis properties in a reproducible and reliable manner [84]. Utilizing microfluidics lets for the same parameters used in small-scale batches to be useful in parallel during the scale-up process.
2.1. Role of temperature controller in nanoparticle synthesis using a microfluidic platform
Now-a-days, the temperature controller module is very essential and plays a vital role in several fields. This is primarily due to its growing applications in every domestic and industrial heating and cooling applications. Further, the temperature management device is extensively used in various domains like nanoparticle synthesis, industrial automation, biomedical, food and agricultural industries, pharmaceutical, and rheological applications [85–87]. The microcontroller based temperature management system can be developed with different heaters such as peltier, cartridge, nichrome wire, polyimide (Kapton), coil-based, and resistive heaters, leading to automation and user-friendliness. Generally, the thermal management system works on the principle of PI and PID controller [88, 89] for better accuracy which can be employed using the microcontrollers such as arduino, nodeMCU, and ESP modules [90].
A PID based thermal management system is widely used because of its accuracy, reliability, and stability. Table 2 shows the comparison of different types of heating elements that can be used for nanoparticle synthesis. Various temperature sensors that can be used for monitoring the desired temperature values can be incorporated with a thermocouple, thermistor, resistance temperature detector (RTD), analog, and digital sensor ICs. The advantages of the thermal management system include cost-effectiveness, portability, easy-to-use, miniaturization, and automation. It is more efficient, operative, and consistent in upholding the preferred temperature range, despite fast changes in ambient temperature, offering precise and accurate measurements when compared to conventional on/off controller. Consequently, a low-cost temperature controller can be developed using fewer electronic components such as a controller unit, self-designed driver circuitry, and data logging modules for controlling and monitoring the desired temperature while synthesizing the nanoparticles. Many researchers have investigated the heat management modeling and thermal flow process using different methods such as electroosmotic flow-based approach for microchannel applications [91, 92].
Tables 1 and 2 illustrate the comparison of various temperature heating elements and summarizes the different temperature sensors pertaining to microfluidic-based devices involved in the synthesis of a nanoparticle. Table 3 depicts the various characterization techniques used in the morphological and elemental analysis of synthesized nanoparticles.
Table 1. Comparison of various temperature heating elements.
| Variables | Peltier | Cartridge | Nichrome wire | Thin-film resistive | Polyimide (Kapton) |
|---|---|---|---|---|---|
| Max. temperature | −60 °C to 160 °C | >350 °C | 1050 °C | 120 °C | 220 °C |
| Power consumption | More | Minimal | More | Low | Low |
| Heat response time | Slow | Fast | Medium | Slow | Fast |
| Measured property | Current | Voltage | Current | Resistance | Resistance |
| Accuracy | Low | High | High | Medium | Medium |
| Heater Cost | High | Low | Moderate | Moderate | High |
| Applications | Portable coolers, consumer products | 3D printer, biomedical and biochemical devices | Power electronic devices | Medical diagnostics and audio equipment's | MEMS & Microfluidics devices |
Table 2. Summary of different temperature sensors.
| Specifications | Thermocouple | Thermistor | RTD | Analog IC | Digital IC |
|---|---|---|---|---|---|
| Temperature range | −270 °C to 1800 °C | −80 °C to 350 °C | −260 °C to 850 °C | −40 °C to 125 °C | −55 °C to 125 °C |
| Stability | Low | Moderate | Best | Moderate | Good |
| Precision | ±0.5 °C | ±0.1 °C | ±0.01 °C | ±2 °C | ±0.2 °C |
| Output type | Voltage | Resistance | Resistance | Voltage or current | Voltage or current |
| Cost | Low | Low | Moderate | High | Low |
Table 3. Comparison of various conventional based nanoparticle synthesis methods.
| Methods | Response Temperature and Time | Size (nm) | Morphology | Solvent | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Co-precipitation | 30 °C–95 °C, a few minutes | 20–200 | Spherical | Water | Narrow size distribution and high production rate | No control over the shape | [50] |
| Hydrothermal | >250 °C, few hours to days | 500–600 | Spherical | Water-ethanol | Very narrow distribution of size and very good shape control | Marginal product, a requirement of high pressure | [93] |
| Microemulsion | 30 °C–60 °C, a few hours | 5–15 | Cubic or spherical | Organic compound | Relatively narrow size distribution, good shape control, a vessel containing a high concentrated solution | Low yield, a surfactant is difficult to remove, a small amount of particles can be synthesized | [48] |
| Laser pyrolysis | 5 °C–40 °C, a few hours | 1200 | Spherical with less large | Organic compound | High production rate | Large aggregates are formed | [94] |
| Sol-gel | Ambient conditions, few hours | 450–600 | Spherical | Water | Atoms of desired shape and length can be produced, useful for making hybrid nanoparticles. | Contains sol-gel matrix components at their surfaces | [95] |
| Thermal Decomposition | 100 °C–300 °C, usually hours to days | 500–550 | Spherical | Organic compounds | Very narrow size distribution, good shape control, and highly scalable | The prerequisite of high temperature and inert atmosphere | [96] |
| Sonochemical | 10 °C–30 °C, | 25 | Spherical and rod-shaped | Water | Particle production is simple | Probability of Sample loss | [97] |
2.2. Materials characterization
The materials characterization plays a very important role in nanoparticle synthesis. In nanotechnology, it is significant for understanding their applications and properties. Here the experimental methods and instruments used for several measurements concerning the characterization of the synthesizing nanomaterials have been discussed. Spectroscopy is a dominant instrument to scrutinize the architecture of organic, inorganic, and nanocrystalline materials. Spectroscopy is one of the methods used to interact energy with samples to execute an analysis. Typically, as an output, the spectrum is plotted with the intensity of energy versus wavelength of energy. Significant variables catalysts, like chemical and texture surface, are a decisive factor that co-relate their catalytic action to their respective properties. Adsorption refers to the existence of a higher concentration of any particular component at the surface of a liquid or a solid phase. The Brunauer–Emmett–Teller (BET) theory is based on an over-simplified model of multilayer adsorption, the BET method continues to be used as a standard procedure for the determination of surface area. Thus, the characterization of catalysts turns out to be the main fact during their analysis. Various techniques are adopted to characterize the nanomaterials are Field emission scanning electron microscopy (FESEM) [98], Energy dispersive x-ray analysis (EDX) [99], Transmission electron microscopy (TEM) [100], X-ray diffraction (XRD) [101], X-ray photoelectron spectroscopy (XPS) [102], UV visible spectroscopy (UV–vis) [103], Brunauer–Emmett–Teller (BET) [104], and Fourier transforms infrared spectroscopy (FT-IR) [105].
Table 4 below summarizes the various characterization techniques used in the morphological and elemental analysis of synthesized nanoparticles. Table 5 shows the Comparison of various conventional based nanoparticle synthesis methods.
Table 4. Different characterization techniques and their applications.
| Characterization techniques | Applications |
|---|---|
| Field emission scanning electron microscopy [106] | To study and analyze the structure, topology, and compositions of the polymers, biological materials, metals, and composites. |
| Energy-dispersive x-ray analysis [107] | To identify the elemental composition of materials. The data produced by the EDX study contain a spectrum which shows peak corresponding to the elements making up the factual composition of the sample being examined. Applications comprise product research and materials, deformulation, and troubleshooting. |
| Transmission electron microscopy [108] | To investigate the local structures, dispersion of multi-component polymers, morphology, crystallization of metallic alloys, the microstructure of composites, and semiconductors. |
| X-ray diffraction [109] | To determine the crystalline properties of solid-state substances. |
| X-ray photoelectron spectroscopy [110] | To examine quantitative chemistry and atomic composition. Also extensively utilized as a surface study method as it can be realistic to a wide range of resources and offers valuable quantitative and chemical state statistics from the surface of the particle being analyzed. |
| UV-visible spectroscopy [111] | To study ionic and molecular species capable of absorbing at UV or visible wavelengths in diluted solutions. |
| Fourier transforms infrared spectroscopy [112] | To scrutinize only molecular compounds such as natural products, organic compounds, and polymers, etc |
| Brunauer–Emmett–Teller [113] | Brunauer–Emmett–Teller (BET) theory aims to explain the physical adsorption of gas molecules on a solid surface and serves as the basis for an important analysis technique for the measurement of the specific surface area of materials. |
Table 5. Summary of comparison between conventional and microfluidic methods.
| Variables | Conventional approach | Microfluidic approach |
|---|---|---|
| Device footprint | Bulky instrument | Miniaturized platform |
| Process/Control | Manual | Automated |
| Power usage | More power consumption | Consumes less power |
| Reaction volume | Millilitres | Microliters to nanoliters |
| Installation | Difficult | Easy-to-use |
| Thermal dissipation | More loss | Less thermal loss |
| Operation platform | Macroscale | Microscale |
| Thermal aspects | The necessity of hot air oven or furnace | Any micro-heater |
3. Conventional based nanoparticle synthesis techniques
Several forms of nanostructures can be produced conventionally through different kinds of the synthesis process. A nanostructure can be defined as a structure with at least one or two measurements in the 1 nm–100 nm range [114]. Nanomaterials can be generated using diverse methods. Dry particles and nanomaterials in fluidic form diffusion are manufactured by these methods. Figure 4 depicts the different methods of nanoparticle synthesis. Nanostructures can be produced by constructing molecules or by fading the size from micro material to nanomaterial [115].
Figure 4. Different techniques involved in conventional nanoparticle synthesis.
Download figure:
Standard image High-resolution image3.1. Co-precipitation
The co-precipitation method is a widely used technique for the generating of nanoparticles in the precipitated phase. In the co-precipitation method, the reactions include the parallel incidence of nucleation, coarsening, growth, and agglomeration procedures. Co-precipitation reveals several features like yields are usually inexplicable classes are designed under circumstances of high supersaturation, nucleation is a fundamental phase, and a huge amount of fine atoms will be formed. The co-precipitation method offers few benefits like simple and quick preparation, ease of control towards nanoparticle size and configuration, low temperature based operation, produces efficient energy, homogeneity, and no requirement of organic solvent [116]. The drawbacks of co-precipitation are it consumes more time, issues in batch-to-batch reproducibility, and not suitable for variable precipitation rates.
3.2. Microemulsion
The microemulsion is one of the processes utilizing ideal technologies for the production of the inorganic nanomaterials, the mechanism involved in the synthesis of the nanoparticle is unique within microemulsions. When the microemulsion particle, considering solvents, are altogether varied, the reagent exchange takes place throughout the impact of liquid droplets in the microemulsion. The microemulsion method has benefits such as ease-of-preparation, thermodynamic stability, minimum agglomeration, the realization of nanomaterials with a regular crystalline arrangement and large specific surface area to volume ratio, and a high grade of nanoparticle size distribution and controllability of configuration [117]. There are few demerits associated with the microemulsion method like restricted solubilizing volume for nanomaterials with thawing facts, exterior aspects such as pH and temperature get influenced by the stability of microemulsions, and the microemulsion formation needs a large volume of surfactant and co-surfactant and these at high concentrations are normally infuriating.
3.3. Ultrasound
The ultrasound method has become a significant tool for nanoparticle synthesis. When fluids are irradiated with ultrasonic based technique is used to form irradiation and cavitation respectively. Ultrasonic cavitation generates a diversity of chemical and physical properties like pressure, high temperature, and cooling rate, which offers an exceptional atmosphere for biochemical activities under stimulating circumstances. Ultrasound is one of the sufficient techniques for nanoparticle production with morphology controllability [118]. The advantages of the ultrasound method are nanoparticles are synthesized at ambient thermal range, simple and fast approach, and nanomaterials can be generated with diverse sizes and shapes, the reaction rate is very high thus, the minimum amount of time is vital to create nanomaterials. The disadvantages include the process being an energy exhaustive process with complex scalability, and heat-sensitive nanomaterials cannot endure acoustic cavitation.
3.4. Microwave-assisted
Microwave-assisted is one of the synthesizing methods which is prevalent in fields ranging from biological procedures to nanomaterials. Chemical activities with reagents are repeatedly quicker than the classical convection process for heating the particles which provide higher yields and fewer side products. Microwave containers offer exceptional mechanisms over reagent blending, enduring high pressures and wide temperature range, and are determined for reproducibility from solution to solution. This method offers improved engineering control over the parting of the nucleation and expansion steps of nanoparticle production when the response process is commenced from the ambient temperature. Microwave-assisted controlled burning offers some selectivity in triggering the chemical compound particles during nanoparticle synthesis, which is significant for scalability [119]. Microwave synthesis has the prospective measure to heat-up the selective precursor or solvent molecules for the preparation of the nanoparticles.
3.5. Sol-gel
The sol-gel method is utilized for creating or generating solid particles from minor molecules. In this biochemical reaction, the sol (or solution) steadily grows towards the creation of a gel-like diphasic arrangement comprising both solid and fluidic phase, the morphologies depict the choice of the process from distinct particles to continuous polymer networks. Unvarying ceramic powders and ultrafine can be designed through a precipitation manner. Above mentioned powders of sole and multi-element configuration can be generated on nano-scale atom size for biomedical and dental applications. In this process, the raw materials are uniformly mixed in the fluidic phase, the polycondensation and hydrolysis reactions are used to form a transparent and stable sol method. The benefits of the sol-gel method can be described as it produces homogeneity, relatively high in purity, porosity controllability, easy to prepare different sizes, and it is a suitable approach for multi-component particles [95]. Some of the disadvantages of the sol-gel method are it is relatively lengthier in response time, and an organic solvent may be injurious to the physical body.
3.6. Hydrothermal
The hydrothermal technique has been well-regarded and has gained attention from various researchers and scientists in the production of nanoparticles aspects. The hydrothermal based synthesis of nanomaterial has been efficacious for the preparation of imperative entities like microporous quartzes, chemical sensing oxides, multifaceted ceramic oxide and fluorides, superionic electrodes, and magnetic resources [120]. It is also a well-established path for inimitable condensed material, comprising nanometer atoms, thin films, gels, with illustrious chiral and helical structures and these are mainly stacking-sequence particles. Hydrothermal synthesis discusses the production of nanoparticles through biochemical solvents in an aqueous solution operated beyond the boiling point of water. Whereas, solvothermal synthesis in a non-aqueous reaction at comparatively high-temperature values. Hydrothermal synthesis involves the generation of chemical reactions of materials in a vacuum-packed thermal reaction above the atmospheric pressure and temperature. The unique properties and morphological investigations of nanostructured graphene particles and catalytic performance of nanostructured particles are discussed from synthesis to physiochemical were evaluated using the hydrothermal method [121, 122]. This method is easier and precise for obtaining regulation over the size and shape distribution dimensions. The crystallinity of the end product via amendment of variables like reaction time and temperature, surfactant and solvent type, and finally the precursor type. The materials with high vapor pressures and low melting points are performed during the nanoparticle synthesis. The hydrothermal synthesis needs expensive autoclaves and lacks safety issues during the reaction process and also there is no transparency while observing the reaction process.
Farahmandjou et al [123], illustrated pure aluminium oxide (Al2O3) and iron-doped alumina Fe-doped Al2O3 with various proportions of Fe dopant nanomaterials were synthesized by co-precipitation approach. To create a pure solution, 18.7 g of Al(NO3)3.9H2O salt was melted in 100 ml of deionized water. Further, the solvent is sonicated at atmospheric temperature for 10 min. Later, the temperature was increased to 75 °C and 5 ml of EG stabilizer was added drip-wise into the reaction mixture and 10 ml of NaOH was added to the solution, after 5 min. Subsequently, the thermal range was raised to 85 °C ensuing in white powder. The yield was washed a few times using ethanol and water to eradicate impurities. To make the impure solution, in a discrete container, 18.5 g Fe(NO3)3.9H2O salt was melted in 100 ml of DI water at atmospheric heat for 10 min beneath magnetic stirrer to acquire uniformity of 0.5 M. Later, Fe dopants of 2%, 4%, and 6% were added to pure alumina. The percentage of Fe in Fe-Al2O3 was organized by regulating the mole ratio of Fe chemical compound in the reaction. The same process is trailed while preparing the pure sample. The XRD characterization shows that the reactions have β, α, γ, and θ stages and the crystal arrangement became more stable with growing Fe dopant. The output of TEM images depicts that the nanoparticle size of the 4%-doped reaction is around 45 nm. The consequences of FTIR studies illustrate a rise in the intensity of the Al-OH concentration with the growth of the Fe dopant. Undoped Al2O3 and Fe-doped Al2O3 nanoparticles were effectively produced with various iron proportions of 2%,4%, and 6% using the co-precipitation approach.
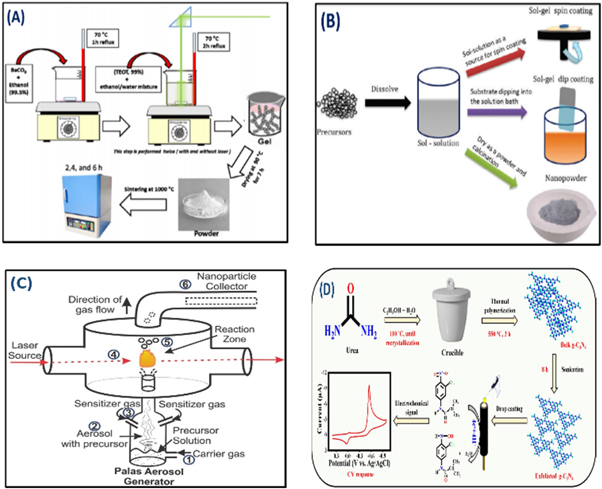
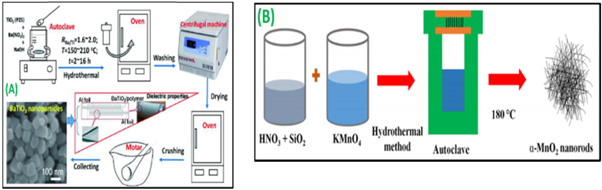
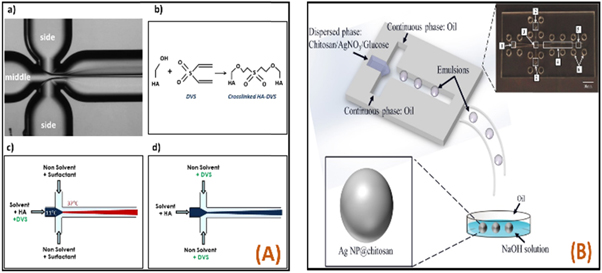
Mostafa et al [124], elucidated the fact on the consequence of distinct laser sources on the microstructure and dielectric possessions of barium titanate (BaTiO3) nanomaterials at various calcination periods throughout the gelling production stage. Here, the nanomaterials were synthesized by the sol-gel technique (figure 5(A)). A couple of laser beams like red and green with wavelength 808 nm and 532 nm respectively were applied precipitously at the center of shaking unprocessed materials. The precursors were agglomerated at 1000 °C for 6 h. The XRD study depicts that the samples arranged under the green laser have maximum transparency. These nanomaterials were described by TEM and FESEM were structural, electrical, and optical properties were analyzed.
Figure 5. (A) Illustration of sol-gel based BaTiO3 nanoparticles synthesis under the effect of a 1000 mW green laser beam [124]. (B) A schematic representation of sol-gel processing [125]. (C) Experimental setup of laser pyrolysis [126]. (D) A schematic approach of g-C3N4 nanoparticle synthesis using the sonochemical process [127]. Reprinted the images from the above-mentioned references with permission of copyright from respective journals.
Download figure:
Standard image High-resolution imageThiagarajan et al [125], discussed the synthesizing of metal oxide nanostructures (MONSs) which show the unique chemical, physical, electronic, biological, biomedical, and optical properties. In this work, the researchers explored an efficient and stable nanoparticle synthesis process which considerably manipulating the efficiency of the MONSs (figure 5(B)). The sol-gel method is used as a technique for synthesizing nanoparticles, this method is one of the simple, economical, and fast protocol processes which has advantages like the requirement of low temperature, product homogeneity, and formation of the complex nanostructures or nanocomposite materials. The sol is acquired by either polymerization or hydrolysis solvents by adding appropriate composition in the chemical compound reaction. Sol liquid can be dropped onto the favored substrates as thin films using a couple of approaches i.e. spin coating and dip coating. ZnO nanomaterials were successfully produced using the sol-gel route were studied using the XRD pattern. In this process, ambient temperature conditions were applied for achieving the thermal strategies during the production of ZnO nanomaterials.
Wang et al [126], explored the novel preparation of N-doped SnO2 nanomaterials through laser-assisted pyrolysis, it is a specific and attractive nanoparticle synthesis method characterized by high flexibility and versatility. The acquired nano-powder was methodically characterized by XPS, HRTEM, and Mossbauer spectroscopy which confirmed the existence of the two phases. SnO2 nanoparticles were successfully synthesized using laser pyrolysis. Throughout the laser-based nanoparticle production, an aerosol of the solvent comprising the chemical compound generated by a nebulizer soared into a container zone with a hauler gas (figure 5(C)). In this container, a CO2 laser beam of wavelength 10.6 μm decomposes the precursors creating nano-powders which are later collected on a fiber nanomaterial produced by laser pyrolysis depicts an encouraging routine as a potential for next-generation lithium-ion batteries (LIBs) negative electrode.
Ganesh et al [127], showed a simple pyrolysis approach followed by the sonochemical method that has been used for exfoliated g-C3N4 synthesis. Herein, sonochemically exfoliated graphite-carbon nitride for the electrocatalyst detection of flutamide in environmental samples. The surface morphology, crystalline arrangement, electrical conductivity, and size distribution were studied using XRD, FTIR, FESEM, and Electrochemical impedance spectroscopy (EIS) (figure 5(D)). Later, the prepared mixture was transmitted into 100 ml Teflon coated autoclave and undisturbed for 12 h at 130 °C. Further, the reaction is collected from the autoclave and washed with ethanol for few times, the acquired slurry was desiccated in a micro-furnace at 50 °C for 6 h and then it was calcined and grinded at 550 °C for 3 h.
Ming Li et al [128], describe the fruition of spherical barium titanate (BaTiO3) nanomaterials with even smaller nanoscale sizes is of excessive importance for the downsized platform for barium titanate based electronic systems. In this study, the authors report on titanium dioxide (TiO2) sowed through the hydrothermal method to produce spherical barium titanate nanomaterials with a size range of 90–120 nm. The BaTiO3 samples were produced through a hydrothermal method employing TiO2 nanomaterials as the Ti seeds and source. Teflon-based autoclaves with a sample volume of 100 ml were utilized as a solvent vessel (figure 6(A)). Typically, NaOH and TiO2 nanoparticles were initially added to 75 ml of DI water and stirred magnetically later Ba(NO3)2 was added to a suspension comprising TiO2 nanomaterials. After prudently sealing, the autoclaves were heated in a hot furnace at 180 °C–220 °C for 16 h. Later, the reaction solution was washed several times. The nanoparticles were further described by several techniques like FESEM and XRD to study and analyze the patterns of the nanostructures. The TiO2 seeded nanoparticles synthesis via hydrothermal technique exhibits a proficient process to produce spherical BaTiO3 nanomaterials for impending capacitor-based energy storage applications.
Figure 6. (A) Schematic diagram showing the synthesis process of α-MnO2 nanowires [128]. (B) Schematic of the hydrothermal synthesis of BaTiO3 nanocrystals using TiO2 (P25) nanoparticles as the seeds and Ti source [129]. Reprinted the images from the above-mentioned references with permission of copyright from respective journals.
Download figure:
Standard image High-resolution imageShah et al [129], demonstrated the production of rectangular nanowires with high purity α-MnO2 nanoparticles were produced by a simplistic single step using the hydrothermal method. The manganese (Mn) and potassium permanganate (KMnO4) were added in 45 ml of deionized water and the precursors were magnetically stirred for 15 min. Throughout the process of stirring simultaneously, 0.5 ml of nitric acid (HNO3) and 0.2 g of silicon dioxide (SiO2) were added to the reaction and further enthused for another 20 min. Further, the reaction was transported to a stainless steel-based vessel called autoclave equipment and heated for 180 °C in an electric oven for 24 h (figure 6(B)). Subsequently the heating process, the samples were washed, sieved, and dried at 120 °C for 4 h in a hot air furnace. Further, the composition and morphology of synthesized α-MnO2 nanoparticles were described by different techniques like XRD, EDX, and Fourier transform infrared (FTIR) for analyzing purposes. The electrochemical possessions of synthesized α-MnO2 nanoparticles have been analyzed as electrode substantial for supercapacitors application. The synthesized α-MnO2 nanowires show a high precise capacitance of 363 Fg−1 at a current concentration of 1.0 Ag−1 with a good cycling steadiness.
4. Microfluidic devices for nanoparticle synthesis
4.1. Microreactor based nanoparticle synthesis:
Synthesizing nanoparticles in the microfluidic environment is one of the most extensively used methods because of its widespread advantages such as easy-to-use, portable, transparent process, micro/nanoscale operation, economical, controllability, and high-throughput during the nanoparticle synthesis. Further, it plays a vital role in producing precise nanostructures with varied size, shape, and morphology of the particles. The microfluidic technology eliminates the need for bulky instruments, being laborious, the essence of expensive autoclaves, and also power-related issues that are associated with the existing conventional methods. The microfluidic platform comprises two types of approaches in the management of fluid flow - a chamber-based approach and a continuous-flow based approach. In the chamber-based method, consists of an inlet and outlet for fluid transformation, the fluid is static or steady placed inside the reservoir or microreactor for a while. Usually, the heater is placed underneath the chamber-based microchannel. Microreactors for the large class of homogeneous liquid-phase solutions are often based on single-phase laminar flow designs in which reagent streams are brought into contact. Here, the working principle is similar to that of the conventional method but operated in a micro-scale environment and portable manner. In a continuous-flow based method, the fluid moves through a narrow microchip concerning the time. The continuous-flow-based microchannel can be of different designs such as serpentine, spiral, and circular respectively.
Kulkarni et al [130], designed a microfluidic platform for synthesizing manganese oxide (MnO2) nanoparticles used for electrochemical sensing applications. The authors have developed a portable and automated thermal management system for the heating process during the nanoparticle synthesis. The PMMA based microchannel (20 mm*20 mm), acting as a microreactor, was fabricated using a CO2 laser ablation technique having a single inlet and outlet (figure 7(A)). The microfluidic device was directly placed onto the copper heating blocks. The rapid and exact desired temperature was achieved and the manganese sulphate and potassium permanganate precursors were taken in 10 ml of DI water stirred for 20 min. The solution of 1 ml of the reaction mixture was transferred to the microchip and the set temperature was around 90 °C for 12 h. The brownish powder was acquired from the process and later, it was clean several times with deionized water and dried by placing the powder in a hot furnace at 60 °C for 3 h. The morphological and size distribution analysis was studied using different characterization methods like FESEM, EDX, and XPS. Further, the obtained MnO2 nanoparticles were used for the electrocatalytic detection of H2O2 and dopamine using the cyclic voltammetry (CV) method to verify the oxidation and reduction peaks.
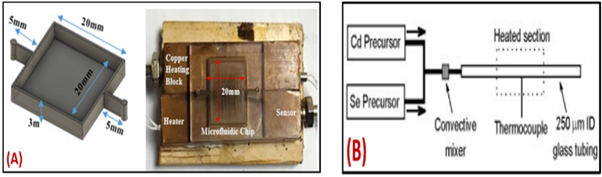
Figure 7. (A) (a) Design of microchannel (b) Microchannel places on the copper block [130]. (B) A schematic representation of the capillary reactor [131]. Reprinted the images from the above-mentioned references with permission of copyright from respective journals.
Download figure:
Standard image High-resolution imageYen et al [131], described a method for synthesizing nanoparticles with the particle size found to be around 2–4.5 nm, and the concentration peak was observed in the range of 450–600 nm. The glass tubing material of dimension 250 μm (inner diameter) was used as a substrate for carrying out nanoparticle synthesis. The convective mixer was fabricated for the proper blending of Cd and Se precursors before moving to the heating section (figure 7(B)). The thermocouple based temperature sensor was used as a feedback signal to control the desired temperature. The reactor was comprised of a miniature convection blender trailed by a heated glass solution microchannel preserved at a steady temperature around 180 °C–300 °C. The total reaction volume was 30 μl. Additionally, cadmium selenide (CdSe) nanocrystals were effectively synthesized by using a microreactor based microfluidic system.
Even though the microreactor-based nanoparticles synthesis is simple, low cost, and easy-to-operate but there are few shortcomings of the microreactor-based nanoparticles synthesis devices such as sample loss, thermal dissipation, and static placement of reaction mixture requires more volume of sample. These parameters can be overcome to a certain extent by using the continuous-flow based nanoparticle synthesis.
4.2. Continuous-flow based nanoparticle synthesis
The continuous-flow based microfluidic device for nanoparticle synthesis is the most widely utilized strategy in the microfluidic platform due to its high level of transparency and controllability where the reaction mixture flowing through the fabricated microchannels. Actuation of reaction flow is realized with the microchannel, by either external mechanical pumps and pressure sources, also with integrated micro-pumps, or by combinations of electro-kinetic mechanisms and through capillary forces. Usually, syringe pumps offer a fast and simple approach for infusing the fluids via microchip inlet and taken outside through an outlet. The continuous-flow microfluidic set-up exhibits easy implementation leading to various applications, and also useful during post-synthesis processes. However, it is less suitable when there is a requirement for a greater degree of flexibility in fluid manipulation. Generally, the closed-channel systems are integrally hard to scale and integrate due to the variables that govern the field of flow along the flow direction, making the fluid flow depending on a single location on the properties of the complete system. Permanent etching of micro-structure also leads to limited potentials to reconfigure the structure. Moreover, substantial volumes of reagents and relatively longer microchannels have to be utilized. In continuous-flow microfluidic devices, highly sensitive and efficient flow sensors are used for increasing the accuracy of the device based on MEMS technology, which is used to monitor the fluid flow in the microchannels. The simplest microreactors used by Maeda et al and Mulvaney et al, for semiconductor nanoparticle synthesis were based on a capillary-tube reactor.
Nightingale et al [132], stated the synthesis of indium phosphide (InP) nanomaterials based on dual inlets and single outlet y-shaped microreactors which provided accurate control over the solution temperature, flow rates, and the reaction time. Microfluidic based containers have recently been raised as ultimate devices for the evolution of high-quality colloidal nanomaterials because of its unique mechanism as they proffer over strategies, and their spectral features are easily used (figure 8(A)). It provides the simplest way of using a microfluidic device comprising a solo capillary dipped in a hot oil-bath, the pre-composed reagents can be determined hydrodynamically. The reaction composition was infused from a syringe pump into a capillary glass having a dimension of 150 μm (ID) and a 40 cm segment of which was immersed in an oil bath at 225 °C. The solution yields were observed at an atmospheric temperature in a glass flow-cell situated at the downstream of the oil-bath, through 335 nm diode-pumped through helium-cadmium (HeCd) laser source and a fiber optic together with CCD spectrometer. The drawback of this system is that because of using a stirrer bar in the oil bath tank there may be a chance of variation in the fluid flow.
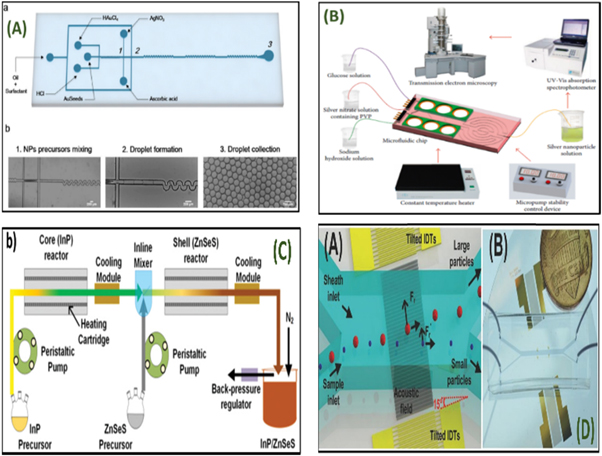
Figure 8. (A) Schematic of quantum dot synthesis using (a) A single capillary (b) A y-shaped microfluidic device [132]. (B) Reactor with a heated solution region and cooled outlet region: a through-etch section ensures that the two sections are thermally insulated [133]. (C) figure 2. Schematic diagram of the installation: 1 - syringe pumps, 2 - mixing module, 3 - nucleation module, 4 - growth module, 5 - a selection of nanoparticles [134]. (D) Configuration set-up for the continuous flow-based production of palladium nanomaterials [135]. Reprinted the images from the above-mentioned references with permission of copyright from respective journals.
Download figure:
Standard image High-resolution imageYen et al [133], demonstrated a simple silicon-based micro-container integrating both liquid-gas segmented flow and multiple thermal regions of the microfluidic system for the production of nanoparticles. Here a series of CdSe nanocrystals with various luminescence colors were synthesized by fine-tuning the ratio of the chemical compound with reaction mixture temperature. The microfluidic device was incorporated with a serpentine-based microchannel with inlets and outlets respectively (figure 8(B)). The heating zone was placed underneath the microchip for achieving precise temperature. There was also thermal insulation at the edges of the outlet for proper heat dissipation. In another experiment, the gas was utilized as a buffer layer to discrete precursor droplets, to curtail the reaction mixture time and to control the accuracy of the nanoparticle size of CdSe quantum dots (QDs). However, such designs are limited in terms of sluggish diffusive reagent fraternization and broad residence time distributions (RTDs).
Mbwahnche et al [134], described an automated way of synthesizing semiconductor and metal nanoparticles using flow reactor synthesis as a new substitute to the batch processing approach of nanomaterial synthesis. Experimental results show the optimal flow rates of reaction mixture droplets. The reactor was successfully applied for the production of colloidal reagents of metal (Ag) and semiconductor (CdSe) nanomaterials (figure 8(C)). The proposed device was applicable both in the material science laboratory and in the synthesis industry. The complete system is operated with the help of a microcontroller arduino mega board and a programmable computer with special software designed in NI LabVIEW for controlling and coordinating the setup. The device consists of syringe pumps, mixing module, nucleation module, growth module, and selection of nanoparticles for the synthesis process. Nevertheless, this instrument is limited to industrial applications.
Gioria et al[135], described a systematic differentiation between batch process and continuous systems for producing palladium nanoparticles (PdNPs). A protocol was established and used for capping reagents and further glucose and starch were used as a reducer respectively. The particle size distribution, morphology, and catalytic action were undertaken for sensing applications. UV–vis, XRD, TEM, and FTIR characterization techniques were used for analyzing and determining the chemical and elemental composition (figure 8(D)). The higher stability of the particle acquired in the microreactor can be confirmed, as the TOF assessment persisted as constant even after 7 months of storage in an aqueous media without thermal or light protection. Flow microreactor leads to minor, monodisperse, and time-stable PdNPs. Here, a comparison was performed between the conventional batch method (round flask reactor) and the continuous flow process (microreactor). After analysis and other considerations on TOF, it was been reported that the use of microreactors has proven to be an excellent method for the generation of time stable palladium nanomaterials by green synthesis. The limitation of this approach is that the use of the tube is not a low-cost polymer and it is unweldable.
Sara abalde et al [136], demonstrated a droplet microfluidics platform for reproducible and highly controlled monodispersed gold nanoparticle synthesis. The device has been automated and integrated by acclimating dualistic distinct bulk imitation strategies to microdroplets, performing on micro-containers, for synthesizing nanoparticles in both surfactant-assisted and surfactant-free manners. Microdroplets were produced in dual discrete microfluidic systems developed to accommodate the necessity of both mass productions (figure 9(A)). The epitaxial development of gold nanostars (AuNSTs) inside the microdroplets endorsed a sufficient control of reagent blending and indigenous concentrations throughout the atom formation process. In this work, the authors reported that this was the first time to synthesize the branched gold nanoparticles in a microfluidic system. The monodispersity of the output was further compared to classical synthesis in mass approach, demonstrating the prospective of this technology for the flow-through based synthesis of high superiority anisotropic nanoparticles with enhanced reproducibility. The monodispersity of the product has the variation to that of synthesis in bulk proving the need for iteration of this technology for tuning of temperature and reaction time for accurate nanoparticle synthesis.
Figure 9. (A) Surfactant free production of AuNSTs in a microdroplets based platform; (a) A simple illustration of the microchip strategy; (b) optical images corresponding to distinct points of the microfluidic chip where numbers depicting diverse areas of the device in (a) resembles optical images in (b) [136]. (B) Schematic illustration of the experimental scheme [137]. (C) Experimental setup of millifluidic reactor for multistep InP/ZnSeS nanoparticles synthesis with dual stages, with cooling modules, and a peristaltic pump system [138]. (D) (A) Schematic approach (B) photograph of the acoustic-based nanoparticle separation device [139]. Reprinted the images from the above-mentioned references with permission of copyright from respective journals.
Download figure:
Standard image High-resolution imageLiu et al [137], described a novel production approach to prepare silver nanomaterials (AgNPs) by utilizing piezoelectric triggered with three-stage flow pulsating blending in a microfluidic chip. The resultant experimental circumstances comprised the concentration of sodium hydroxide and sinking reagent composition, using a dosage of polyvinylpyrrolidone (PVP), with an inlet flow rate and synthesis temperature range. The produced silver nanoparticles were characterized by TEM and UV–vis absorption spectrophotometer (figure 9(B)). The experimental output illustrated the spherical silver nanoparticles with a typical nanoparticle diameter of around 29 nm, with acceptable morphology, high yield, and good monodispersity were generated using a microfluidic chip. The synthesizing temperature of the microfluidic chip was 80 °C. The preliminary concentration of silver nitrate was 1 mM, the concentration ratio of sodium hydroxide to silver nitrate was 2:1, and the concentration ratio of glucose to silver nitrate was 4:1. The optimal flow rate of the microchip was about 3.5 ml min−1. The authors revealed that this method showcased good efficiency and controlled synthesis of nanoparticles was achieved. The proposed device needs other equipment such as micro-pump stability control makes the process, thus it is costlier and difficult to operate for minuscule synthesis.
Nette et al [138], illustrated a method using luminescent and plasmonic parameters that are varied accordingly for synthesizing the photonic nanomaterials using microfluidic reactors which makes high-quality products. Further enabling integrating analytics and real-time solution control which is an emerging powerful tool eases material exploration and reaction parameter mapping, with efficiency and rapidity which is not possible in classical flask-based approaches. Herein, the microfluidic production of photonic nanomaterials, where the luminescent and plasmonic properties are varied and have been reported to be of great potential in automated multidimensional solution factor screening employing in situ visual characterization. In this approach, superfast mixing is achieved where the reaction is initiated by temperature, as a time delay can be introduced between mixing and heating, further to ensure perfect mixing before the reaction starts (figure 9(C)). For instance, the microfluidic setup consists of core and shell synthesis of InP/ZnSeS nanomaterials accessing a two-step continuous flow reactor with integrated cooling and heating modules and with an inline mixer.
Mengxi Wu et al [139], illustrated a unique process for the separation of nanoparticles using acoustic waves in a continuous-flow microfluidic environment. The separation of nanoscale particles was carried out on their differences in size and shape which is an essential method to the nanotechnology and nanoscience community. Here, the authors have demonstrated the successful separation of nanomaterials in a continuous-flow by using tilted-angle standing surface acoustic waves. Herein, a tilted-angle standing surface acoustic wave (taSSAW) device is placed underneath the microchannel which enables size-based nanomaterial filtration, but to further analysis, the fine-tuning of input power and the flow rate was taken-up for separation of 500 nm and 110 nm polystyrene particles. The standing waves cause periodic pressure fluctuations in the liquid within the microchannel. The input power was fixed at 27 dBm, the flow rates of the sample and sheath flow were 5 and 10 μl min−1, respectively (figure 9(D)). The reaction mixture of 500 nm and 110 nm particles passed through the microdevice and further, it was fractionated to various outlets. The bright-field and fluorescent images were recorded at the outlet of the microchannel to manifest the separation performance. The acoustic field induced by taSSW generates a much larger deflection for the 500 nm particles. The motion of 100 nm particles is dominated by the Stokes drag force. The difference in the lateral displacement of particles with different volumes enables size-based separation. The continuous separation of nanomaterials is demonstrated with a ≈90% recovery rate. The acoustic nanoparticle separation method is versatile, non-invasive, and simple.
Bressan et al [140], demonstrated a 3D printed microfluidic device based on fused deposition modeling (FDM) used formed using poly(lactic acid) filament. The poly(methyl methacrylate) slide, allowing the conception of transparent microchannels and is utilized on the flow-through synthesis of gold and silver nanomaterials. Further, to reduce the fouling inside the microchip the device was optimized to use a segmented flow of fluorinated oil. The produced nanomaterials were characterized by UV-Visible spectroscopy, scanning microscopy, and transmission electron microscopy (figure 10(A)). The silver nanomaterials were synthesized using discrete concentrations of sodium borohydride and flow rates of reactants at 20 °C, with sizes ranging from 5 ± 2 nm to 8 ± 3 nm, and verified to be stable for at least two weeks. Gold nanomaterials were produced at 90 °C varying the concentration of trisodium citrate and flow rates of reactants, yielding sizes from 20 ± 9 to 34 ± 12 nm and verified to be stable for a couple of weeks. Later, the gold nanomaterials were employed in surfaced amended Raman scattering using crystal violet as a model molecule.
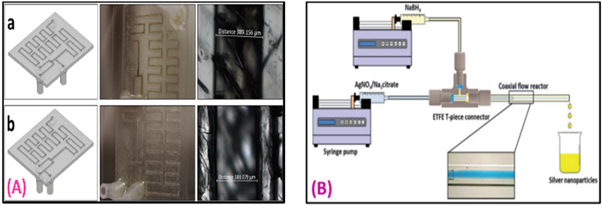
Figure 10. (A) (a) Left) The bottom view of the device is used to produce nanomaterials with the fluorinated oil inlet put after the reactants. Center) Top view photograph of the actual microchip showing fouling along the microchannels. Left) Optical microscopy of the bottom of the microchannel displaying a darker color because of fouling. (b) Left) The device presents the fluorinated oil inlet between the reactants flows. Center) The device offers no fouling along the microchannels. Left) No darker coloration inside the channel due to fouling [140]. (B) Schematic of the coaxial flow reactor setup [141]. Reprinted the images from the above-mentioned references with permission of copyright from respective journals.
Download figure:
Standard image High-resolution imageBaber et al [141], described the production of silver nanomaterials in a coaxial flow reactor (CFR). Such nanomaterials were examined by restraining the solution and ensuing nucleation to a commune aside from the microchannel. The silver nanomaterials were molded at the atmospheric thermal range by decreasing the silver nitrate with sodium borohydride during the existence of hydroxide, meanwhile, trisodium citrate was utilized as the surfactant (figure 10(B)). The authors described the study of the flow rate through the CFR and reactions of silver nitrate and trisodium citrate. During the experimentation, syringe pumps were utilized to convey the dual streams of silver sodium borohydride and nitrate/trisodium citrate solution and reaction to the CFR. The CFR comprised a diminutive inner tube that has been injected into the center of the bigger outer tube. Here, both the inward and outward tubes were designed for glass. The capillaries were coupled together using a T-piece connector and CFR functioned using laminar flow reign for complete experiments.
Russo et al [142], designed a microfluidic platform for hyaluronic acid nanoparticles (cHANPs) for biomedical applications especially to enhance the MRI imaging techniques for the diagnostic purposes that have focussed on the use of nanostructures that entrap magnetic resonance imaging without the necessity of chemically modified and clinically approved compounds. Here, the polymeric based microfluidic system was used to produce crosslinked cHANPs in which a clinically relevant MRI-CAs, gadolinium diethylenetriamine penta-acetic acid (Gd-DTPA), which is been entrapped (figure 11(A)). This approach facilitates a high degree of control over particle generation, enabling the synthesis of monodisperse particles with dimensions of 35 nm. A simple T-shaped microchannel was designed and fabricated where, the reaction was passed into the channel and further, the microchannel was heated up to 37 °C for synthesizing the hyaluronic acid-based nanoparticles. A crosslinking reaction of hyaluronic acid (HA) groups with divinyl sulfone (DVS). The advanced microfluidic environment is suitable for producing the intravascularly-injectable and completely biocompatible hydrogel nanomaterials entrapping clinically approved processes that enable the implementation of straightforward and scalable strategies in therapy and diagnostics applications.
Figure 11. (A) Representation of Microfluidic experimental set-up. (a) Optical Fluorescence Microscopy Image of Flow-Focusing pattern; (b) Crosslinking reaction of HA hydroxyl groups with divinyl sulfone (DVS). Qualitative Illustration of two diverse crosslinking plans processed in our microfluidic device: (c) when DVS is added into the middle channel; (d) when DVS is added into the side microchannels [142]. (B) Representation of synthesis of Ag NP-loaded chitosan amalgamated micromaterials [143]. Reprinted the images from the above-mentioned references with permission of copyright from respective journals.
Download figure:
Standard image High-resolution imageYang et al [143], presented a novel approach to realize a polymer-based microfluidic platform for a single-step and in situ production of silver nanomaterials loaded chitosan microparticles. The proposed method was used for obtaining monodisperse and uniform silver nanoparticles-loaded chitosan microparticles measuring numerous hundred micrometers (figure 11(B)). The diameter of the composites was tuned by altering the flow on the microfluidic chip. Further, the composite nanomaterials containing silver particles were characterized using SEM and UV–vis spectra. Due to the properties of silver NPs and chitosan, further, these generated composite microparticles can be used in many applications such as bactericidal agents for water disinfection, surface plasma resonance enhancers, and antipathogens.
Bhattacharya et al [144], developed a nanoparticle system to efficiently target two receptors overexpressed in the microenvironment of assertive tumors. Herein, hyaluronic acid was regioselectivity improved using a multistep synthetic method to change binding stipulations for P-selectin and CD44 to tumor cell interface. Here, the dual-targeting plan uses sulfate variations on hyaluronic acid that target P-selectin, in addition to intrinsic targeting of CD44, the expression patterns of these couple of receptors in cancer sites. This method can also realistic to analyze a wide range of tumor types expressing both the target, considering the active microenvironment of peak cancers. In this study, the authors also demonstrated that altered hyaluronic acid nanoparticles effectively and efficiently target both CD44 and P-selectin cells, which places the preliminary work for in vivo biomedical applications.
In another study, Bhattacharya et al [145], illustrated an approach where deacetylation of the N-acetyl cluster and demanding sulfation on the C6-OH on the hyaluronic acid-based polymer, which forms serious interactions with the CD44 dynamic site. The altered hyaluronic acid nanoparticles were synthesized, characterized, and analyzed and their interactions were assessed by in vitro and molecular modeling methods. In-vitro methods comprised of fluorescence polarization and, flow cytometry while in-silico methods involved binding and docking calculations by an MM-PBSA methodology. The results of this investigation signify the primary step to operative retargeting of HA-derived nanoparticles for drug delivery and bioimaging.
Table 5 summarizes the several difference between the existing conventional method and microfluidic strategy involved in nanoparticle synthesis used for different applications.
5. General applications of metal oxide nanoparticles
Metal oxide nanoparticles (MNOPs) can be beneficial in numerous fields extending from color pigments, cosmetics, photovoltaics, drug delivery, photocatalysis, and screen coatings. Diverse general applications of metal oxide nanoparticles are discussed here.
5.1. Biomedical applications
Metal oxides nanoparticles, due to their special optical, magnetic, and electrical properties, are more popular for biomedical, biomedicinal, and healthcare applications. These include self-cleaning layers, bio-separation, delivery of a drug, biosensing, bioimaging, antimicrobial, and antibacterial respectively [146, 147]. Nanotechnology products have become progressively useful in the biomedicine and clinical domain and have led to the advent of a hybrid discipline known as nanobiotechnology. Nanoparticles have noteworthy applications in nano biomedical, predominantly in prostheses, diagnosis, bone substituting implants, and drug delivery systems [148]. Nanoscale particles integrate well into biomedical devices because most biological systems are also nanosized. The materials normally utilized to develop these nanotechnology products are inorganic and metal nanoparticles, carbon nanotubes, liposomes, and metallic surfaces [149]. Targeted drug delivery is a significant biomedical application that intends to deliver anticancer drugs to the precise site of the tumor and evade damage to adjacent healthy cells. Presently, iron oxide nanomaterials are the key source of magnetic particles, used to provide anticancer drugs to target specific zones [150]. Another main biomedical application of nanoparticles in the treatment of hyperthermia. Magnetic hyperthermia treatment cures tumors by heating them to around 45 °C to abolish the cancerous cells. The advantage of this method over chemotherapy is that it explicitly targets the tumor and does not damage the surrounding fine tissue. Again iron oxide (Fe3O4) nanomaterials are the main material that is currently used for this treatment [151].
Nevertheless, other nanosystems like bimetallic nanomaterials Cu–Ni, Fe–Co, and magnetic nanomaterials like Co–Fe2O4, Mn–Fe2O4, and Ni–Co2O4 have also been investigated [152]. In recent times, biosensors have appeared as a significant biomedical application for detecting a variety of biomolecules and cell culture. A variety of nanomaterials, such as Au, Fe–Pt, CeO2, and TiO2, have been investigated for conceivable use in biosensors [153]. The use of magnetic nanoparticles as contrast agents for bioimaging methods like computed tomography and magnetic resonance imaging is another key biomedical application of nanoparticles [154]. Figure 12 shows the schematic of a few important biomedical applications of nanoparticles.
Figure 12. Schematic representation of a few biomedical applications of nanoparticles.
Download figure:
Standard image High-resolution imageThe MONPs are extensively utilized in tissue engineering for generating biological and biochemical alternatives of organs and tissues in mammals [155]. Moreover, the MONPs exhibit an absorbent framework by the creation of responsive oxygen classes like hydrogen peroxide (H2O2) and superoxide (O2-) which can be utilized in adhesion, wound healing, angiogenesis, propagation, and cell function. In a few studies, various MONPs have been examined, for instance, TiO2 nano coral-based structural design offers great controllability over drug discharging device under ultraviolet (UV) light source for cancer chemotherapy application. Similarly, ZnO nanoparticles demonstrate superior antibacterial, antimicrobial, anticancer, bioimaging, biosensing, and outstanding UV blocking properties. SnS2 nanoparticles can be utilized for its antibacterial and antimicrobial properties. Tungsten oxide nanoparticles can be a decent substituting particle for non-mediated biosensors for electrochemical applications [156]. Hyaluronic acid (HA) is a comparatively new polymer for the construction of drug delivery systems [157]. Cellular acceptance and the efficacy of tumor-targeted drug delivery are hypothetical to upsurge by utilizing hyaluronic acid as drug carriers [158].
5.2. Photocatalysis applications
Metal oxide nanoparticles can be used to create photogenerated charge carriers upon inflammation with an essential quantity of light-dependent energy and having technological importance in ecological remediation and electronics. The metal oxide nanoparticles like TiO2, NiO, MnO2, and ZnO have been utilized as photocatalysts because of their governable electronics and optical properties [159]. Several metal oxide-based nanoparticles are examined for their usage in the self-cleaning, antibacterial, and deodorization method. For instance, the garbage water can be filtered by removing injurious bacteria and other carbon-based contaminants with the semiconductor photocatalysis route [160].
5.3. Artificial photosynthesis
Artificial photosynthesis is a biochemical procedure that imitates the natural procedure for photosynthesis, a method that transforms light source energy, water, and CO2 to fuel sources. An artificial photosynthesis scheme promptly generates fuels from light that could offer a methodology to accessible energy storage and a field of technology for the carbon-neutral generation of high-energy concentration for fuel conveyance. Numerous doped and undoped photo-electrodes have been discovered by investigators to produce such coordination using MONPs like ZnO, CuO2, MnO2, and TiO2 and WO3. Though these metal oxides create firm photo-anodes, their huge bandgaps can only absorb an inadequate portion of light. NiO2 and TiO2 have revealed ideal protection for the photo-anodes as described in a few literature [161–165]. Nevertheless, there are some other inorganic semiconductors like CdTe, InP, and Si which can also do a similar task with an ultra-thin shielding coat of metal oxide which is catalytic, non-porous, and have suitable interfacial energetics.
5.4. Gas sensing applications
MONPs are the most surveyed and suitable particles for the gas sensor because of their great surface reactivity and large surface area to volume ratio [166]. One of the problems for MONPs gas sensors is to accomplish high selectivity. Numerous MONPs have been utilized for decreasing, detecting the combustible or oxidizing concentration of gases by perceiving electrical conductivity. In the interaction with the nearby ambient variations, the conductivity of metal oxide increases due to its functionality associated with the electrical signal is communicated to the electrode for better performance. Hereby, an indirect way of heating is implemented for attaining a change in electrical conductivity. WO3 and SnS2 have extended antiquity in sensing applications, particularly in gas sensors domains [167, 168].
5.5. Photovoltaics applications
The photovoltaic strategies exploit semiconductor-based metal oxide nanoparticles. These exhibit outstanding elasticity for their optoelectronic properties and ease of preparation technique dealing with metal oxides which offer an exclusive candidate for the next-generation photovoltaic devices. Moreover, their outstanding competence to a sphere or recover the system characteristics allows indispensable applications in the field of the flexible solar cell, printable electronics, semi-transparent devices. Metal oxide nanoparticles are widely utilized for diverse tenacities in solar cells [169]. For example, an electron-hole blocking layer/transfer layer and dye-sensitized solar cells (DSSCs) and mesoporous scaffolds in perovskite solar cells (PSCs). TiO2 nanoparticles are one of the most extensively utilized resources for photovoltaic applications. Further, the devices designed on a flexible substrate, ZnO is a favored entrant due to its advanced electron mobility in contrast with TiO2 nanoparticles [160, 170]. Besides, a low-temperature nanomaterials synthesis does not require a heating and sintering process for its operation.
5.6. Hydrogen fuel generation
Now-a-days adaptation of solar energy into hydrogen signifies a challenging and attractive platform for the generation of hydrogen fuel, thus an alternative path for photovoltaic solar cells. The production of hydrogen fuel has gained a wide range of applications in automotive industries for generating power sources in automobiles. In 1972, a crucial invention was noticed in the area of photocatalysis which was initiated from fujishima and honda motors where they discovered the electrolysis of water and the progression of the hydrogen gas-based vehicles. Thereafter, several nanoparticles have been seen which act as a photocatalyst to generate hydrogen fuel by persuading photocatalyst in the fluid. Numerous metal oxide semiconductors were utilized to rift the liquid into oxygen and hydrogen upon luminosity. The most candid approach to generate hydrogen from sunlight and aquatic sources is by connecting an electrolyzer to a solar cell assortment. SnO2 and TiO2 own great chemical firmness over a wide variety of pH and applied capabilities. Also, these days, nanotechnology is gaining great attention in enhancing oil recovery (EOR) based nanoparticles because of its cost-effective nature and environmentally, friendly manner, and have the potential to reduce the oil viscosity, increase the mobility ratio, and to alter the reservoir permeability [171, 172].
5.7. Energy storage and harvesting applications
Recently, supercapacitors have become an epicenter to grab attention due to their usage in the storage of energy. The charge storing contrivance of a supercapacitor powerfully relies on the superficial properties of the electrode materials. These nanoparticles retain a high surface area to volume ratio alongside great surface energy, but the combination of nanomaterials is one of the challenging problems. To overwhelm this issue mesoporous nanoparticles are extensively utilized for the fabrication of supercapacitors electrode. Mesoporous nanoparticles like mixtures of metal oxides of vanadium pentoxide (V2O5) and TiO2 are active as electrode substantial for the supercapacitor because of its exceptional physical and chemical properties. Many studies are described for metal oxide nanoparticles based electrodes in supercapacitors. For instance, Sasirekha et al [173], produced ZnO nanoparticles through the sol-gel technique, layered with carbon (ZnO/C) to organize a probe for supercapacitor, unveiling a precise capacitance of 92 F g−1. Similarly, Bonu et al [174], investigated the role of SnO2 nanoparticles and SnS2 quantum dots incorporated in carbon microspheres which exhibited precise capacitance as high as 420 F g−1 [175]. Furthermore, it can be used for energy harvesting purposes for diverse applications such as fuel cells [176] and triboelectric nanogenerators [177].
The crosslink enhanced emission (CEE) in a new sort of non-conjugated polymer dots (PDs). The enhanced PL originates from the decreased vibration and rotation of amino-based chromophores. Furthermore, the cellular acceptance mechanism and internalization of PDs were examined in detail [178]. In another study, the chemical crosslinking of the sub-fluorophore, linear non-conjugated polymers can retain strong photoluminescence (PL), which is a very significant fluorescence behavior and the non-conjugated polymer dots (PDs) are effective bio-fluorophores for bio-based applications [179].
Table 6 summarizes the simple comparison of various methods used for synthesizing nanoparticles in microfluidic strategy.
Table 6. Comparison of different methods used for synthesizing NPs in Microfluidic environment.
| Microfluidic approach | Design | Method | Synthesized NPs | Benefits | References |
|---|---|---|---|---|---|
| Chamber-based | Micro-reactor | Hydrothermal | MnO2 NPs | Low cost, IoT enabled device | [130] |
| Continuous-flow based | Straight | Co-precipitation | Polymeric NPs | Simple and flexible tubing | [180] |
| Serpentine | Turkevich | Au NPs and Hg2+ detection | LOD - 10−6 M (0.2 ppm) | [181] | |
| Spiral | Co-precipitation | Hollow spherical silica | Scalable flow synthesis strategy | [182] | |
| Droplet | Wet-chemical | Anisotropic Metal Nanocrystals | Flexibly fine tune NPs | [183] |
6. Conclusion
In the recent past, there has been a drastic swift in the synthesis of nanoparticle methods and approaches where a shift from the conventional method of synthesis to the microfluidic platform is gradually leading the nanoparticle synthesis. In this review, some of the recent work accomplished in both the conventional approach and in the microfluidic strategy has been discussed and summarized. Also, the innovative microfluidic systems, materials, and fabrication processes involved in the design and development of microfluidic devices have been deliberated. With a variety of outputs from the devices, by variable flow rates, reactant concentrations, and thermal aspects, microfluidic technology generally allows the synthesis of diverse monodisperse nanomaterials like metallic, nanocomposites, polymers, and semiconductors of preferred size and shape. The chamber-based and flow-through based microfluidic devices have been successfully utilized for generating the nanomaterials of desired nanostructures and specific characteristics. The chamber-based methods are extensively utilized for nanomaterials production due to their simplicity, reproducibility, easy mode of operation, and reliability during synthesizing nanoparticles, which has a smaller footprint of the microchip. The continuous-flow based methods are also being utilized for the production of nanoparticles as they exhibit controllability and high-throughput. The review also discusses droplet-based microreactors which can overcome a few limitations of the method and how the high-quality nanomaterials are successfully synthesized. The nanoparticle synthesis results obtained from microfluidic devices show a similar output when compared to that of conventional methods in terms of appearance, homogeneity, and electrochemical sensing applications. Also, the results showed a considerable improvement in the narrow size distribution of nanoparticles. Usually, at a high volumetric fluid drift rate, the narrow size distribution was originated as the average residual time is inversely proportional to the fluid transfer rate. Also, microfluidic devices offer more robust and are reliable benefits, and can be integrated with a large diversity of laboratory equipment for synthesizing on-chip functionality.
Despite remarkable progress towards microfluidic-based synthesis, their development will always evolve in parallel with conventional or well-established methods. Further, the nanoparticle synthesized via these two distinct methodologies will find diverse applications in nanotechnology and biotechnology. Eventually, the microfluidic-based nanoparticle synthesis will become a method of choice only for applications that mainly depend on the exceptional properties of materials. Such an outlook resembles to be an encouraging platform for microfluidic synthesis of nanomaterials, together with the large-scale conventional methods. Such an approach manifest wide opportunities for future scope in the development of innovative techniques using microfluidic technology for the generation of nanomaterials. The future developments of microfluidic devices in synthesizing nanomaterials will be oriented towards large-scale applications by using multiple simultaneous microfluidic reactors.
Acknowledgments
The authors are thankful to Center for Human Disease Research (CHDR), BITS-Pilani, Hyderabad Campus for providing the financial support during this work. Also, Madhusudan B Kulkarni is thankful to Tata Consultancy Services (TCS) for providing scholarship during this project.