Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Authors: Vanessa Ngan, Staff Writer, 2004. Updated: Dr Kelvin Truong, Dermatology Research Fellow, Westmead Hospital, Sydney, Australia; Dr Martin Keefe, Dermatologist, Christchurch, New Zealand. Copy edited by Gus Mitchell. September 2021.
" data-index="1" alt="Go to Introduction
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(1 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Introduction
" data-index="2" alt="Go to Uses
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(2 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Uses
" data-index="3" alt="Go to Contraindications
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(3 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Contraindications
" data-index="4" alt="Go to More information
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(4 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">More information
" data-index="5" alt="Go to Benefits
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(5 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Benefits
" data-index="6" alt="Go to Disadvantages
" onclick="event.preventDefault();document.getElementsByTagName('h2')[(6 - 1)].scrollIntoView({behavior: 'smooth',block: 'start'});">Disadvantages
Side effects and risks
Tacrolimus is a macrolide calcineurin inhibitor immunosuppressant drug available as a topical ointment, oral capsule, and intravenous injection. It was initially isolated from the soil fungus Streptomyces tsukabaenis.
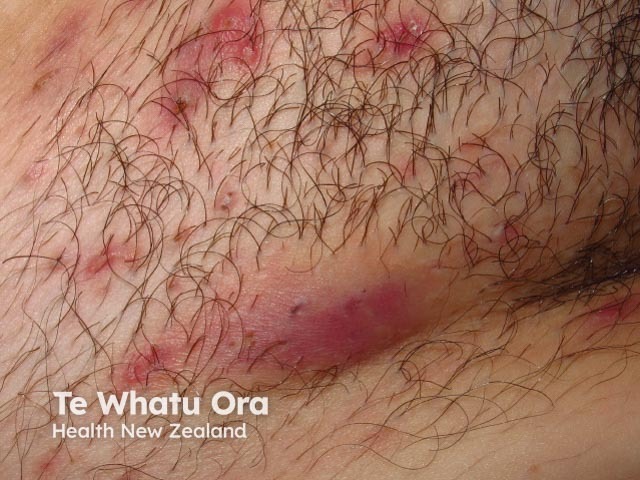
Hidradenitis suppurativa
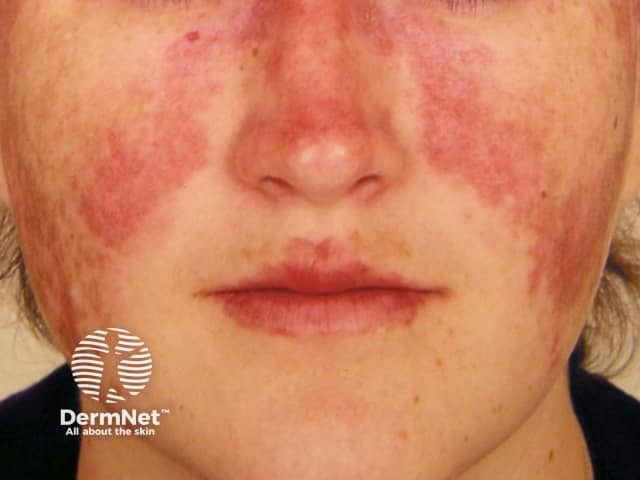
Systemic lupus erythematosus
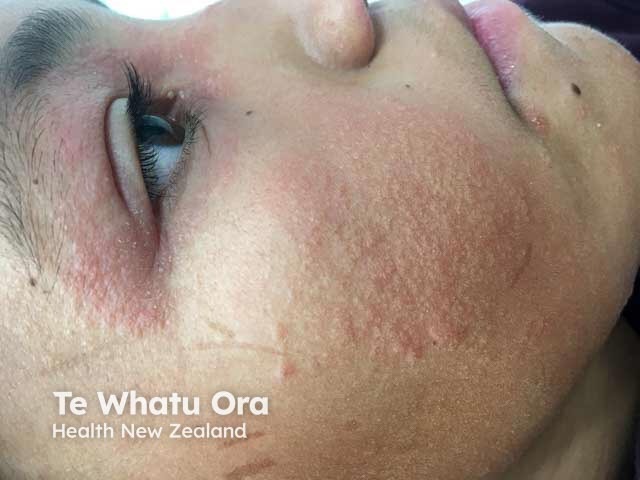
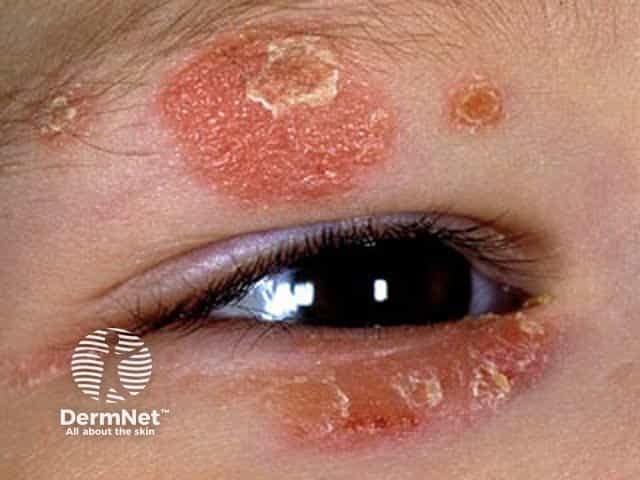
Face and eyelid atopic dermatitis in a child
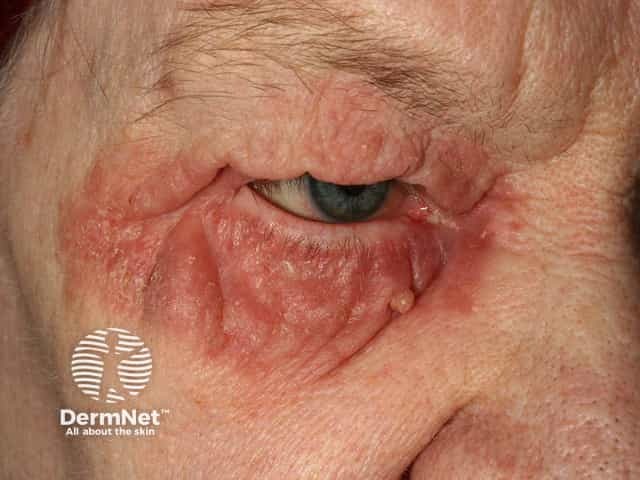
Seborrhoeic dermatitis
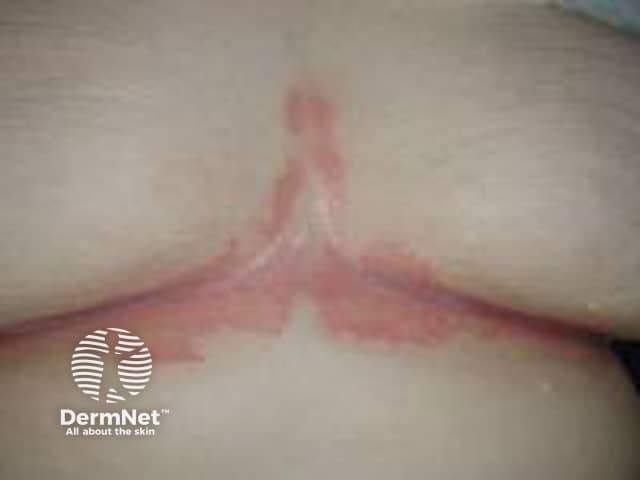
Flexural psoriasis
Facial psoriasis
Tacrolimus is a lipophilic molecule; for topical use it is formulated as an ointment. Percutaneous absorption is minimal except where there is an epidermal barrier defect such as in active atopic dermatitis. As disease activity settles, absorption through the skin reduces.
Tacrolimus is metabolised by cytochrome P450 in the liver. Tacrolimus is not metabolised in the skin.
Polymorphisms in the CYP3A5 gene affect the bioavailability of systemic tacrolimus with CYP3A5 non-expressors requiring a higher dose of systemic tacrolimus post-transplant.
Tacrolimus suppresses the cell-mediated immune response.
The Food and Drug Administration (FDA) has a black box warning for a possible increase in cancer risk including skin cancers and lymphoma. Evidence is conflicting with topical use so the risk, if any, is probably very small.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).