Abstract
Blood vessels and nerves often follow parallel trajectories, suggesting that distal targets use common cues that induce vascularization and innervation. Netrins are secreted by the floor plate and attract commissural axons toward the midline of the neural tube. Here, we show that Netrin-1 is also a potent vascular mitogen. Netrin-1 stimulates proliferation, induces migration, and promotes adhesion of endothelial cells and vascular smooth muscle cells with a specific activity comparable to vascular endothelial growth factor and platelet-derived growth factor. Our evidence indicates that the netrin receptor, Neogenin, mediates netrin signaling in vascular smooth muscle cells, but suggests that an unidentified receptor mediates the proangiogenic effects of Netrin-1 on endothelial cells. Netrin-1 also stimulates angiogenesis in vivo and augments the response to vascular endothelial growth factor. Thus, we demonstrate that Netrin-1 is a secreted neural guidance cue with the unique ability to attract both blood vessels and axons, and suggest that other cues may also function as vascular endothelial growth factors.
The patterns of the neural and vascular networks are highly conserved within and between species. The identification of neural guidance cues by means of genetic screens and in vitro assays demonstrates that specific programs are hard-wired to pattern the nervous system (1-3). These programs consist of guidance cues that signal through neuronal cell surface receptors and induce axons to either extend toward or away from the source. There are four major classes of neural guidance cues: ephrins, semaphorins, slits, and netrins (2). Ephrins, semaphorins, and slits are ligands that bind to cognate receptors and repulse the distal tip of an axon or growth cone. Netrins were the first neurite attractants identified, and are secreted by the floor plate, where they function to attract spinal commissural axons toward the midline of the neural tube (4, 5). Extensive crosstalk between signaling pathways enables nerves to integrate multiple guidance instructions (6, 7). Thus, through the combinatorial regulation of relatively small numbers of attractive and repulsive cues, axons are directed to form the intricate and highly reproducible neural circuitry.
Our understanding of angiogenesis has evolved from the identification of potent vascular mitogens, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF), to an investigation of how endothelial cells and vascular smooth muscle cells (VSMCs) are patterned to form an integrated network of blood vessels (8-13). Similar to the neural network, the vascular network forms from central axial structures that send sprouts along predetermined trajectories to their distal destinations. The trajectories of nerves and blood vessels are often shared, leading to the hypothesis that tissues may use similar if not identical factors to instruct both their innervation and vascularization (14, 15). Indeed, recent evidence indicates that neuronal guidance factors such as semaphorins, ephrins, and slits and their receptors function as angiogenic regulators. First, gene-targeting experiments have demonstrated that both ephrinB2 and EphB4 are essential for angiogenesis (16). Second, semaphorin controls vascular morphogenesis by functionally interacting with integrin receptors and the receptor Plexin-D1 to regulate angiogenesis in zebrafish and mice (17-19). Finally, the interaction of slit with its receptor, Robo, modulates vascular endothelial cell migration (20, 21).
Of the four major families of axonal guidance factors, the netrin family has yet to be implicated in vascular biology. The netrins comprise a family of at least three proteins (Netrin-1, -2, and -4) that have significant structural similarities with the laminin family of extracellular matrix proteins (4, 5, 22). Netrin-1 is the most extensively studied gene in the netrin family (4, 5). In addition to its role in axon guidance, recent studies strongly suggest that Netrin acts outside the neuronal system; for example, in lung, mammary gland, and pancreatic development (23-25).
Axons can be directed to their correct destination by neural guidance factors that regulate the cytoskeletal architecture and filopodial extensions of the growth cone. Blood vessels are guided toward their appropriate target by migration and proliferation of constituent vascular cells. Here, we provide evidence that netrin can function as a growth factor for the vasculature. We show that Netrin-1 stimulates proliferation, induces migration, and promotes adhesion of endothelial cells and VSMCs. We also show that Netrin-1 stimulates angiogenesis in vivo and augments the response to VEGF. Together, our data demonstrate that Netrin-1 is a neural guidance cue with the unique ability to attract blood vessels as well as axons, and is capable of functioning as a vascular growth factor.
Experimental Procedures
Expression Studies. Total RNA was isolated from human umbilical vein endothelial cells (HUVEC), human microvascular endothelial cells (HMVEC), and human aortic VSMCs by using TRIZOL (GIBCO/BRL). Human brain RNA was purchased from BD Biosciences. The RNA was reverse-transcribed with the RETROscript kit (Ambion) according to the manufacturer's instructions. The primer pairs for human DCC were 5′-ACAGGCCTCAAACCAAACAC-3′ (forward) and 5′-ACCTCCATCTCCATGACGAC-3′ (reverse). Those for human neogenin were 5′-ACCCCAGCCTGTGATTAGTG-3′ (forward) and 5′-TGTGATGGTTCAGAGCTTGC-3′ (reverse). The primer pairs for GAPDH were 5′-ACCCAGAAGACTGTGGATGG-3′ (forward) and 5′-TGCTGTAGCCA A ATTCGTTG-3′ (reverse). The following conditions were adopted for amplification of DCC and neogenin: denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 45 seconds, 35 cycles. The conditions used to amplify GAPDH were denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 45 seconds, 30 cycles.
Immunohistochemistry and Western Blot Analysis. Immunohistochemistry and Western blot analyses were performed as described (20, 26). Primary antibodies that recognize Netrin-1 (Oncogene), Deleted in Colorectal Cancer (DCC) (Oncogene), Neogenin (Santa Cruz Biotechnology), Unc5H2 (two different antibodies: gifts from Lindsay Hinck, University of California, Santa Cruz; polyclonal antibodies were raised against a GST-ectodomain of Unc5h2 fusion protein), and CD31 (Dako) were used in these studies.
In Vitro Assays. BSA and PDGF-BB were obtained from Sigma. Recombinant human VEGF-165, FGF-2, and Netrin-1 (mouse and chicken) were purchased from R & D Systems. Fibronectin, Laminin-1, Collagen I, and Collagen IV were from BD Biosciences. Human aortic VSMCs, human aortic endothelial cells (HAEC), and HMVEC were cultured according to supplier's instructions (Cambrex, East Rutherford, NJ). Rodent aortic VSMCs were isolated as described (27). All cell biology assays were performed by individuals blinded to treatments and repeated on at least two independent vials of primary cells, with each experiment reproduced three separate times, and each condition within an experiment performed in triplicate.
We used the most direct measurement of mitogenic activity, that is, cell number. Low passage primary endothelial cells or VSMCs were seeded onto 24-well plates at equal density and serum-starved for 16 h. After starvation, factors (BSA, PDGF-BB, VEGF-165, and Netrin-1) were added to the media (EBM containing 0.5% FBS and 0.1% BSA for endothelial cells; DMEM containing 0.1% BSA for VSMCs) at indicated concentrations. After 24, 48, and 72 h of treatment, cell number was measured with a hemacytometer or by counting fixed and stained cells in the well. Fold increase over BSA-treated wells was used to compare different cell types, time points, and test factors. Representative fields of cells stained with hematoxylin and eosin (Sigma) were photographed.
To measure migration, vascular endothelial cells were serum-starved overnight and then seeded at a density of 40,000 cells per well onto 8-μm Transwell inserts (Costar). Test factors were added in serum-free media and placed in the lower chamber. After incubation at 37°C for 3 h, filters were fixed with Zamboni's fixative and stained with a 4′,6-diamidino-2-phenylindole (DAPI) stain kit (Fisher Scientific). The total number of migrated cells was calculated by counting five random fields; this number was then expressed as fold increase. VSMC migration was performed as described above, except 30 ng/ml PDGF-BB was used as the positive control. For blocking experiments, cells were pretreated with 0.1 μg/ml of antibodies that recognize either Neogenin or DCC for 30 min before addition of cells to the upper chamber.
To measure adhesion, 96-well plates were coated with BSA, Netrin-1, Fibronectin, Laminin-1, Collagen I, or Collagen IV for 16-20 h at 4°C. Wells were blocked with PBS containing 1% BSA for 1 h at room temperature before the addition of cells. Cells were harvested by trypsinization and neutralized with growth media, washed twice, and then resuspended in serum-free DMEM containing 0.5% BSA. A total of 5 × 104 cells were added to each well and allowed to attach for 30 min at 37°C in a CO2 incubator. After washing three times with PBS, cells were fixed, stained with hematoxylin and eosin, and counted. For blocking experiments, cells were pretreated for 30 min with 10 μg/ml of neogenin antibody.
In Vivo Assays. Chorioallantoic membrane assay (CAM) was performed as described (28, 29). Briefly, fertilized Leghorn chicken eggs were incubated under conditions of constant humidity (60%) at 37°C for 72 h. On days 3-4, eggs were opened into sterile clingwrap hammocks and incubated at 37°C with 2.0% CO2 and 90% relative humidity for 4 more days. At days 7-8, test samples were diluted 1:1 in sterile 1.0% methylcellulose and applied to 1-mm3 Gelfoam sponges (Upjohn). The sponges were overlayed with a 3 × 3 mm 250-μm-pore nylon mesh (Sefar America, Depew, NY) and cured on 13-mm Thermanox coverslips (Nalge Nunc), for 2 h at room temperature under sterile conditions. Assembled test samples were placed on the CAMs and incubated at 37°C for a treatment period of 72 h. The CAMs were then fixed in 4% paraformaldehyde/2% glutaraldehyde/PBS and photographed on a Leica MZ 12.5 equipped with a Zeiss Axiocam digital camera. The average number of microvessels surrounding the perimeter of the implanted mesh in each image was calculated by image j (NIH image) or a blinded reviewer and expressed as fold increase over BSA-treated CAMs.
For the murine corneal micropocket assay, hydron (Hydro Med, Cranbury, NJ) pellets containing sucralfate (Sigma), and the indicated test factors were prepared and implanted as described (30, 31). Briefly, 7- to 8-week-old male C57BL/6 mice were anesthetized with i.p. avertin (Sigma). The eyes were topically anesthetized with proparacaine, and an incision was made lateral to the pupil with a no. 10 surgical blade. A corneal micropocket was dissected from this incision toward the limbus with a von Graefe knife no. 3. A pellet was implanted in this micropocket, and topical erythromycin was applied. Five to six days after pellet implantation, neovascularization was quantitated by visualization with a slit lamp microscope. The neovascularized area was computed with the formula 2π/10 × clock hours × vessel length (mm).
Results
To determine whether Netrin-1 is a vascular mitogen, we measured cell number after addition of Netrin-1 to primary endothelial cells or VSMCs. We compared the responses of endothelial cells to Netrin-1 versus VEGF, a known endothelial mitogen. As shown in Fig. 1, primary HAEC and HMVEC proliferate in response to Netrin-1. Netrin-1 elicited the same level of proliferation as VEGF in both cell types, although the response of HMVEC consistently exceeded that of HAEC (Fig. 1A). Dose-response experiments revealed that the activity of Netrin-1 peaked at 0.7 nM (50 ng/ml) and produced similar levels of endothelial proliferation as 0.6 nM (10 ng/ml) VEGF (Fig. 1B). Netrin-1 also serves as a mitogen for VSMCs, producing similar levels of proliferation as PDGF, a known VSMC mitogen (Fig. 1 C and D). In contrast to endothelial cells, the mitogenic activity of Netrin-1 on VSMC did not peak at 50 ng/ml. Direct cell count was confirmed by using the nonradioactive colorimetric assay WST-1, and [3H]thymidine assays confirmed stimulation of DNA synthesis (data not shown).
Fig. 1.
Netrin-1 stimulates proliferation of vascular endothelial cells and VSMCs. The mitogenic potential of Netrin-1, compared to known vascular growth factors, was assessed by measuring the increase in cell number of cultured endothelial cells (A and B) and VSMCs (C and D) after application of these factors to the culture medium. The data are presented as fold increase in cell number relative to control cells that were treated with BSA in serum-free media. Each panel represents the results of at least three independent experiments, each performed in triplicate. (A) After 48 h of treatment, Netrin-1 (50 ng/ml, 0.7 nM) and VEGF (10 ng/ml, 0.6 nM) stimulated proliferation to a similar extent in both primary HMVEC and HAEC. (B) HMVEC exhibit a dose-dependent response to Netrin-1. Treatment of HMVEC with increasing concentrations of Netrin-1 reveals a peak mitogenic activity comparable to that of VEGF at an intermediate concentration of 50 ng/ml. (C and D) Netrin-1 is a mitogen for VSMC exhibiting a potency similar to, or exceeding, that of PDGF. (C) After 48 h of treatment with either Netrin-1 (50 ng/ml, 0.7 nM) or PDGF (30 ng/ml, 2.5 nM), a 2-fold increase in the number of primary rat or human VSMC was observed compared to BSA-treated (control) cells. (D) VSMC exhibits a dose-dependent response to Netrin-1. Cellular proliferation is enhanced with increasing concentrations of Netrin-1. Asterisks indicate treatments that yield a statistically significant increase (P < 0.05, Student's t test) in proliferation. The results are expressed as mean ± SD.
To assess the capacity of Netrin-1 to induce migration, we measured the movement of cells through a filter toward a chamber filled with test factors. First, we compared the response of endothelial cells to Netrin-1, VEGF, and BSA. Fig. 2 A and B shows that Netrin-1 induces HAEC and HMVEC migration in a dose-dependent fashion. The migration of endothelial cells toward Netrin-1 was directional, or chemotactic, because eliminating the gradient between the two chambers blocked migration (Fig. 2C). Second, we showed that Netrin-1 was chemotactic for human and rat VSMC (Fig. 2 D-F) with a potency similar to PDGF. As observed with the mitogenic properties of Netrin-1, there were subtle differences between the responses of endothelial cells and VSMC, as VSMC migration to Netrin-1 peaked at 50 ng/ml. When nonvascular cell types such as NIH 3T3 or COS cells were used in our cell culture assays, they did not respond to Netrin-1 (data not shown). Thus, Netrin-1 is a potent mitogen and chemoattractant for both endothelial cells and VSMC with a specific activity comparable to VEGF or PDGF.
Fig. 2.
Netrin-1 induces migration of endothelial cells and VSMCs. A modified Boyden chamber assay was used to assess the ability of Netrin-1 to stimulate endothelial cells (A-C) and VSMCs (D-F) to migrate across a porous filter, compared to the known chemoattractants VEGF and PDGF, respectively. The data are presented as the fold increase in migration observed with the test factor relative to the control cultures treated with BSA. The figures represent the results of at least three independent experiments, each performed in triplicate. (A-C) Netrin-1 is chemotactic for endothelial cells with a comparable specific activity to VEGF. (A) Netrin-1 at 50 ng/ml (0.7 nM) and VEGF at 10 ng/ml (0.6 nM) each induced similar degrees of HAEC and HMVEC migration in a Boyden chamber. (B) HMVEC cell migration is enhanced with increasing concentrations of Netrin-1. (C) Netrin-1 induces a directional migration, as suggested by the loss of migration when equamolar concentrations are present in both the upper and lower chambers. (D-F) Netrin-1 is chemotactic for VSMC with similar, or greater, specific activity than PDGF. (D) Netrin-1 at 50 ng/ml (0.7 nM) and PDGF at 30 ng/ml (2.5 nM) each induced migration to a comparable extent in either rat or human VSMC. (E) Dose-response curves for Netrin-1-induced VSMC migration identified the peak activity around 50 ng/ml. (F) Netrin-1 induces a directional migration, because an equamolar concentrations of Netrin-1 in each chamber reduces VSMC migration. The asterisk indicates treatments that yield a statistically significant increase (P < 0.05, Student's t test) in migration. The results are expressed as the mean ± SD.
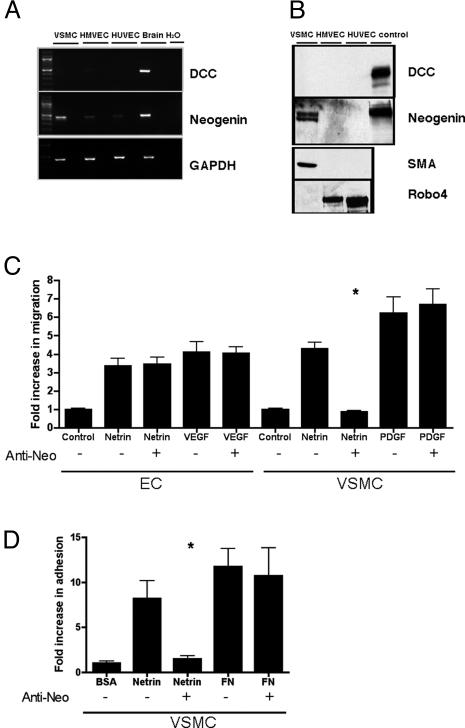
Netrin-1 has significant structural similarities to the laminins, proteins known to promote cellular adhesion (24). To test cellular adhesion, we coated cell culture wells with BSA, Netrin-1, or extracellular matrix proteins with known adhesive properties. Vascular cells were placed on the coated wells and washed after 30 min. The number of cells that remained attached to the well was counted. We found that VSMC, but not endothelial cells, adhere to Netrin-1 (Fig. 3). Moreover, Netrin-1 promoted VSMC adhesion as effectively as other vascular matrix proteins, such as Laminin-1, Collagen I, and Collagen IV. Thus, in contrast to migration and proliferation assays in which endothelial cells and VSMC both respond to Netrin-1, there is a sharp distinction between the ability of endothelial cells and VSMC to adhere to Netrin-1.
Fig. 3.
Netrin-1 promotes adhesion of VSMCs, but not endothelial cells. Quantitative analysis of the adhesion of VSMCs and endothelial cells (EC) to Netrin-1 is shown. The wells of cell culture dishes were coated with test matrix elements or Netrin-1. EC and VSMC were seeded on the treated wells for 30 min, followed by PBS wash. Adhesion was quantified by comparing the number of cells that adhered to a test coating relative to BSA-coated wells. The images represent the results of at least three independent experiments each performed in triplicate. (A) Primary rat and human VSMC adhered to Netrin-1 and fibronectin (FN), whereas HAEC and HMVEC did not adhere to the Netrin-1, but bound tightly to FN. (B) Representative fields of hematoxylin and eosin-stained wells of human EC and VSMC from adhesion assays. (C) Adhesion of VSMC to Netrin-1 is comparable to FN, Laminin-1, Collagen I, and Collagen IV. The asterisk indicates treatments that yield a statistically significant increase (P < 0.05, Student's t test) in adhesion. The results are expressed as the mean ± SD.
Axonal attraction by Netrin-1 is mediated by DCC receptor family members that include DCC and Neogenin (2). To determine whether these receptors are involved in the vascular response to Netrin-1, we first examined whether mRNA or protein was detected in either HMVEC or human VSMC. We failed to detect DCC transcript or protein in endothelial cells by using RT-PCR, Northern blot, or Western blot analysis (Fig. 4 A and B and data not shown). However, we were able to detect a neogenin transcript and gene product in VSMC, (Fig. 4 A and B and data not shown). In a specific subset of nerve cells, Netrin-1 can function as a repulsive cue, and this activity is mediated by a heterodimer comprised of DCC and a coreceptor, Unc5H2 (7). Although we have confirmed reports by others that Unc5H2 is expressed during murine embryonic angiogenesis (32), we did not detect expression of Unc5H2 in endothelial cell or VSMC lines by Northern or Western blot analysis. By RT-PCR, a faint amplified product for Unc5H2 was detected only after 35 cycles (data not shown). These expression data suggest that vascular expression of Unc5H2 may be different in mouse embryonic development versus the adult human cell lines. We examined whether Neogenin mediates Netrin-1 signaling in VSMC by using both migration and adhesion assays. Functional blocking Neogenin antibodies inhibited Netrin-1-mediated migration and adhesion of VSMC (Fig. 4 C and D). The effect was specific, because these antibodies failed to block either PDGF-induced migration of VSMC or Netrin-1-induced migration of primary endothelial cells that do not express neogenin. Thus, Neogenin mediates Netrin-1 signaling in VSMC.
Fig. 4.
Neogenin mediates netrin signaling in VSMCs. The expression and functional contribution of the previously reported Netrin-1 receptors, DCC and Neogenin, to Netrin-1-induced migration and adhesion were assessed in endothelial cells and VSMC. (A) Neogenin was detected in VSMC, and to a much lesser degree in HMVEC and HUVEC, via RT-PCR analysis. DCC receptor expression was not detected in either endothelial cells or VSMC. Human brain RNA served as a positive control for detection of both receptors. (B) Antibodies that recognize DCC, Neogenin, the endothelial cell marker Robo4, and smooth muscle cell actin (SMA) were used in Western blot analysis of primary endothelial cell and VSMC lines. Cell lines known to express the designated gene products were used as controls. Neogenin protein, but not DCC, was detected in VSMCs. Neogenin and DCC were not detected in either endothelial cell type. Antibodies that recognize SMA and the endothelial-specific receptor Robo4 confirmed the identity of VSMC and endothelial cells, respectively. (C) Neogenin mediates VSMC migration to Netrin-1. Neogenin-blocking antibodies inhibited the migration of VSMC to Netrin-1. Conversely, this antibody did not block endothelial cell migration or PDGF-mediated VSMC migration. (D) Neogenin mediates VSMC adhesion to Netrin-1. Neogenin blocking antibodies inhibited netrin-mediated VSMC adhesion and had no effect on fibronectin-mediated VSMC adhesion. The asterisk indicates treatments that give a statistically significant decrease (P < 0.05, Student's t test) in migration or adhesion. The results are expressed as the mean ± SD.
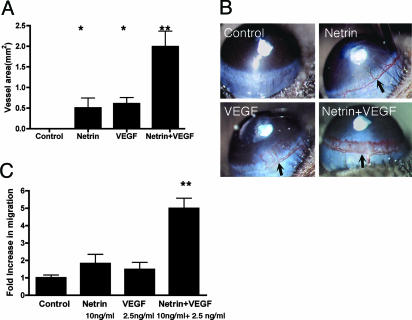
To determine whether Netrin-1 functions as an angiogenic factor in vivo, we measured the response of blood vessels to Netrin-1. In the first model, gelfoam sponges soaked in 1 μg of BSA, Netrin-1, FGF, or VEGF were placed on the chorioallantoic membrane of chick yolk sacs, and the number of vessels invading these sponges was measured after 72 h. Netrin-1 and VEGF each stimulated a 2-fold increase in angiogenesis over BSA control sponges (Fig. 5). In a second model, hydron pellets with equimolar amounts of either Netrin-1 (200 ng) or VEGF (50 ng) were placed on murine corneas and induced equivalent angiogenic responses after 5 days (Fig. 6 A and B). Control pellets elicited no blood vessel formation. Thus, Netrin-1 can function as an angiogenic factor in both in vivo and in vitro assays. Interestingly, when equimolar amounts of the two factors were applied together, the response was robust and greater than the sum of the responses to each of the factors individually. To examine the relationship of Netrin-1 and VEGF in our in vitro assay, low doses of Netrin and VEGF were applied in a migration assay. Migration to 10 ng/ml Netrin-1 or 2.5 ng/ml VEGF was <2-fold relative to the BSA control. However, when Netrin-1 and VEGF were combined, the response was greater than the sum of each factor individually (Fig. 6C). Together, these data suggest that Netrin-1 and VEGF may act synergistically to induce angiogenesis both in vitro and in vivo.
Fig. 5.
Netrin-1 induces angiogenesis in vivo CAM assay. Netrin-1 induces vascular sprouting in a chick CAM assay. Sponges soaked with 1 μg of Netrin-1, VEGF, FGF, and BSA were placed on chick CAMs. The number of vessels sprouting into the sponges after 72 h was counted and quantified as the fold increase over BSA-treated sponges (control). For each test factor, a total of 24 CAM assays were performed. Quantitative assessment and visual representations of each treatment are shown. The asterisks indicate treatments that yield a statistically significant increase (P < 0.05, Student's t test) in angiogenesis.
Fig. 6.
Netrin-1 is synergistic with VEGF in both the in vivo corneal micropocket assay and the in vitro migration assay. (A and B) Netrin-1 induced angiogenesis in a murine corneal micropocket assay. Hydron pellets containing equimolar amounts of either Netrin-1 (200 ng) or VEGF (50 ng) stimulated comparable levels of blood vessel growth. When combined, the two factors generated an accentuated response that was greater than the sum of the responses to each factor individually. In a single experiment, each test factor was placed on six corneas. Each experiment was repeated a minimum of three times. Quantitative assessment and visual representations are shown. (C) Treatment of endothelial cells with Netrin-1 and VEGF together synergistically enhances migration. Either 10 ng/ml Netrin-1 or 2.5 ng/ml VEGF alone showed a <2-fold increase over BSA control. However, when 10 ng/ml Netrin-1 and 2.5 ng/ml VEGF were combined, the response was greater than the sum of each factor treated separately. The asterisks indicate treatments that yield a statistically significant increase (P < 0.05, Student's t test) in angiogenesis. The double asterisks indicate treatment that yields a statistically significant increase (P < 0.001) over individual Netrin-1- or VEGF-treated samples.
Discussion
The superimposable patterns of blood vessels and nerves suggests that distal targets might secrete factors that attract both vessels and nerves (14, 15). Here, we show that the neural attractant, Netrin-1, has a previously undescribed role in promoting angiogenesis. First, cell culture experiments demonstrate that Netrin-1 specifically stimulates proliferation, migration, and adhesion of vascular cells with potencies comparable to VEGF, PDGF, or Fibronectin. Second, Neogenin mediates netrin signaling in VSMC. The lack of expression of the known netrin receptors such as DCC, Neogenin, or Unc5H family members suggest that these factors do not mediate the proangiogenic effects of Netrin-1 on endothelial cells. Recent data indicate a role for integrins in Netrin-1-mediated adhesion (24); however, Netrin-1 does not promote endothelial adhesion, and blocking integrin signaling has no effect on the response of endothelial cells to Netrin-1 (data not shown). Together, these data suggest that an unidentified netrin receptor activates endothelial cell proliferation and migration. Different netrin receptors for endothelial cells and VSMCs may explain the differences in the response of these two cell types to Netrin-1 in migration and adhesion assays. Finally, the chorioallantoic membrane and the murine corneal micropocket experiments demonstrate that Netrin-1 activates blood vessel growth in vivo. Thus, here we report a canonical neural guidance factor that attracts axons and promotes angiogenesis in vitro and in vivo.
Our data strongly suggest a role for Netrin-1 in angiogenic growth stimulation. We found that netrin-1 is also expressed in nonneural tissues during the early stages of murine embryonic development when angiogenesis is underway. High levels of netrin-1 mRNA are evident within the developing somites that flank intersomitic vessel tracts (data not shown). Given these results, one might predict that early vascular development would be defective in embryos lacking netrin-1. To determine whether Netrin-1 plays a role in developmental angiogenesis, we examined two model systems in which netrin activity was attenuated. First, we examined murine embryos that were homozygous for a strong hypomorphic allele of netrin-1 at embryonic days 9.5 and 15 and postnatal day 0.5, but found no evidence of primary cardiovascular abnormalities. Similarly, morpholino knockdown experiments of Netrin-1a and 1b in zebrafish (the only known netrin orthologues in zebrafish) did not alter cardiovascular development (data not shown). There are two possible explanations for these observations. First, there are at least three netrin family members, netrin-1, netrin-2, and netrin-4, as well as two genes encoding Netrin-G1 and Netrin-G2. These proteins have all been implicated in directing axonal projections, although only mice lacking netrin-1 have been described (5, 22, 33-36). Thus, functional redundancy in the vascular system is plausible, and generation of multiple mutant mice, and intercrosses thereof, will be required to assess the roles of netrin family members in embryonic vascular development. A second explanation for the apparent lack of an embryonic vascular phenotype in netrin-1 hypomorphs is the possibility that there are differences in the potency of netrin-1 between adult and embryonic vascular cells. Examination of adult tissues revealed that Netrin-1 protein is expressed within and surrounding blood vessels (data not shown). The data we present herein were derived entirely from adult endothelial cells and VSMCs. To address the possibility of differences between embryonic and adult cells, it will be necessary to purify embryonic endothelial cells and assess their behavior to Netrin-1 treatment directly.
Not only does Netrin-1 induce angiogenesis, it augments the angiogenic activity of VEGF in both in vitro and in vivo systems (Fig. 6). Recent reports indicate that VEGF is a neurotrophic factor, suggesting that the combination of VEGF and netrin family members may work in synergy to induce the vascularization and innervation of distal targets. Although none of the other neural guidance cues are known to have mitogenic activity individually or in combination with VEGF, there is evidence that axonal repulsive cues such as semaphorins, slits, and ephrins either inhibit endothelial migration or block angiogenesis (17, 20, 37-39). Thus, it is likely that neural attractive and repulsive cues, and pro- and antiangiogenic peptides, may act as shared guidance signals that coordinate the patterning of parallel networks such as blood vessels and nerves, and potentially epithelial tubes (23, 25). If guidance signaling programs share regulatory factors in both vascular and neuronal systems, we would expect that, in certain biological contexts, netrins may also act as repulsive or antiangiogenic agents. This expectation is based on evidence that, in axonal guidance, netrin can be bifunctional, mediating attraction and repulsion, dependent on expression and heterodimerization of the DCC and Unc5H2 receptors (7). Future studies will be needed to determine whether netrins have dual roles in vascular guidance.
Due to their therapeutic potential, there is considerable interest in the identification of new vascular mitogens. The major families of vascular mitogens include multiple genes and isoforms of the VEGF, PDGF, and FGF families. The biologic and therapeutic roles of these factors have been studied extensively (40, 41). The recent identification of endocrine gland-selective VEGF, EG-VEGF, raises the possibility that new classes of tissue-specific endothelial mitogens may await discovery (42). Similarly, we would not be surprised if Netrin-1 is simply the first of many neural guidance factors to induce axonal projection and to function as a potent vascular mitogen. The potential for treating ischemic vascular diseases, cancer, or retinopathy by targeting netrins is evident and needs to be explored. To fully realize this potential, the identity of the endothelial receptor responsible for the angiogenic activity of Netrin-1 and the interplay between each netrin family member with other angiogenic peptides and neural guidance factors must be defined.
Acknowledgments
We are grateful to C. Jones and Drs. K. Whitehead and B. Wilson for their assistance and advice and to Drs. L. Urness and K. Thomas for helpful comments and discussions on the manuscript. D.Y.L. is supported by grants from the National Institutes of Health and the American Cancer Society. C.J.K. is supported by National Institutes of Health Grant 1 R01 CA95654-01 and is a Burroughs Wellcome Foundation New Investigator in the Pharmacological Sciences and a Kimmel Foundation Scholar in Translational Science.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; HUVEC, human umbilical vein endothelial cells; HMVEC, human microvascular endothelial cells; HAEC, human aortic endothelial cells; CAM, chorioallantoic membrane; VSMC, vascular smooth muscle cell.
References
- 1.Tessier-Lavigne, M. & Goodman, C. S. (1996) Science 274, 1123-1133. [DOI] [PubMed] [Google Scholar]
- 2.Yu, T. W. & Bargmann, C. I. (2001) Nat. Neurosci. Suppl. 4, 1169-1176. [DOI] [PubMed] [Google Scholar]
- 3.Song, H. & Poo, M. (2001) Nat. Cell Biol. 3, E81-E88. [DOI] [PubMed] [Google Scholar]
- 4.Serafini, T., Kennedy, T. E., Galko, M. J., Mirzayan, C., Jessell, T. M. & Tessier-Lavigne, M. (1994) Cell 78, 409-424. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy, T. E., Serafini, T., de la Torre, J. R. & Tessier-Lavigne, M. (1994) Cell 78, 425-435. [DOI] [PubMed] [Google Scholar]
- 6.Stein, E. & Tessier-Lavigne, M. (2001) Science 291, 1928-1938. [DOI] [PubMed] [Google Scholar]
- 7.Hong, K., Hinck, L., Nishiyama, M., Poo, M. M., Tessier-Lavigne, M. & Stein, E. (1999) Cell 97, 927-941. [DOI] [PubMed] [Google Scholar]
- 8.Yancopoulos, G. D., Davis, S., Gale, N. W., Rudge, J. S., Wiegand, S. J. & Holash, J. (2000) Nature 407, 242-248. [DOI] [PubMed] [Google Scholar]
- 9.Folkman, J. & D'Amore, P. A. (1996) Cell 87, 1153-1155. [DOI] [PubMed] [Google Scholar]
- 10.Folkman, J. & Klagsbrun, M. (1987) Science 235, 442-447. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara, N. (2002) Nat. Rev. Cancer 2, 795-803. [DOI] [PubMed] [Google Scholar]
- 12.Senger, D. R., Galli, S. J., Dvorak, A. M., Perruzzi, C. A., Harvey, V. S. & Dvorak, H. F. (1983) Science 219, 983-985. [DOI] [PubMed] [Google Scholar]
- 13.Shing, Y., Folkman, J., Sullivan, R., Butterfield, C., Murray, J. & Klagsbrun, M. (1984) Science 223, 1296-1299. [DOI] [PubMed] [Google Scholar]
- 14.Mukouyama, Y. S., Shin, D., Britsch, S., Taniguchi, M. & Anderson, D. J. (2002) Cell 109, 693-705. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet, P. (2003) Nat. Rev. Genet. 4, 710-720. [DOI] [PubMed] [Google Scholar]
- 16.Wang, H. U., Chen, Z. F. & Anderson, D. J. (1998) Cell 93, 741-753. [DOI] [PubMed] [Google Scholar]
- 17.Serini, G., Valdembri, D., Zanivan, S., Morterra, G., Burkhardt, C., Caccavari, F., Zammataro, L., Primo, L., Tamagnone, L., Logan, M., et al. (2003) Nature 424, 391-397. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Vazquez, J., Gitler, A. D., Fraser, S. D., Berk, J. D., Van, N. P., Fishman, M. C., Childs, S., Epstein, J. A. & Weinstein, B. M. (2004) Dev. Cell 7, 117-123. [DOI] [PubMed] [Google Scholar]
- 19.Gitler, A. D., Lu, M. M. & Epstein, J. A. (2004) Dev. Cell 7, 107-116. [DOI] [PubMed] [Google Scholar]
- 20.Park, K. W., Morrison, C. M., Sorensen, L. K., Jones, C. A., Rao, Y., Chien, C. B., Wu, J. Y., Urness, L. D. & Li, D. Y. (2003) Dev. Biol. 261, 251-267. [DOI] [PubMed] [Google Scholar]
- 21.Wang, B., Xiao, Y., Ding, B. B., Zhang, N., Yuan, X., Gui, L., Qian, K. X., Duan, S., Chen, Z., Rao, Y. & Geng, J. G. (2003) Cancer Cell 4, 19-29. [DOI] [PubMed] [Google Scholar]
- 22.Koch, M., Murrell, J. R., Hunter, D. D., Olson, P. F., Jin, W., Keene, D. R., Brunken, W. J. & Burgeson, R. E. (2000) J. Cell Biol. 151, 221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan, K., Strickland, P., Valdes, A., Shin, G. C. & Hinck, L. (2003) Dev. Cell 4, 371-382. [DOI] [PubMed] [Google Scholar]
- 24.Yebra, M., Montogomery, A. M. P., Diaferia, G. R., Kaido, T., Silletti, S., Perez, B., Just, M. L., Hildbrand, S., Hurford, R., Florkiewicz, E., et al. (2003) Dev. Cell 5, 695-707. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Y., Stein, E., Oliver, T., Li, Y., Brunken, W. J., Koch, M., Tessier-Lavigne, M. & Hogan, B. L. (2004) Curr. Biol. 14, 897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urness, L. D., Sorensen, L. K. & Li, D. Y. (2000) Nat. Genet. 26, 328-331. [DOI] [PubMed] [Google Scholar]
- 27.Karnik, S. K., Brooke, B. S., Bayes-Genis, A., Sorensen, L., Wythe, J. D., Schwartz, R. S., Keating, M. T. & Li, D. Y. (2003) Development (Cambridge, U.K.) 130, 411-423. [DOI] [PubMed] [Google Scholar]
- 28.Ergun, S., Kilik, N., Ziegeler, G., Hansen, A., Nollau, P., Gotze, J., Wurmbach, J. H., Horst, A., Weil, J., Fernando, M. & Wagener, C. (2000) Mol. Cell 5, 311-320. [DOI] [PubMed] [Google Scholar]
- 29.Ekstrand, A. J., Cao, R., Bjorndahl, M., Nystrom, S., Jonsson-Rylander, A. C., Hassani, H., Hallberg, B., Nordlander, M. & Cao, Y. (2003) Proc. Natl. Acad. Sci. USA 100, 6033-6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenyon, B. M., Voest, E. E., Chen, C. C., Flynn, E., Folkman, J. & D'Amato, R. J. (1996) Invest. Ophthalmol. Vis. Sci. 37, 1625-1632. [PubMed] [Google Scholar]
- 31.Kuo, C. J., Farnebo, F., Yu, E. Y., Christofferson, R., Swearingen, R. A., Carter, R., von Recum, H. A., Yuan, J., Kamihara, J., Flynn, E., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 4605-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelkamp, D. (2002) Mech. Dev. 118, 191-197. [DOI] [PubMed] [Google Scholar]
- 33.Wang, H., Copeland, N. G., Gilbert, D. J., Jenkins, N. A. & Tessier-Lavigne, M. (1999) J. Neurosci. 19, 4938-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, J. C., Ho, W. H., Gurney, A. & Rosenthal, A. (2003) Nat. Neurosci. 6, 1270-1276. [DOI] [PubMed] [Google Scholar]
- 35.Nakashiba, T., Nishimura, S., Ikeda, T. & Itohara, S. (2002) Mech. Dev. 111, 47-60. [DOI] [PubMed] [Google Scholar]
- 36.Serafini, T., Colamarino, S. A., Leonardo, E. D., Wang, H., Beddington, R., Skarnes, W. C. & Tessier-Lavigne, M. (1996) Cell 87, 1001-1014. [DOI] [PubMed] [Google Scholar]
- 37.Prasad, A., Fernandis, A. Z., Rao, Y. & Ganju, R. K. (2004) J. Biol. Chem. 279, 9115-9124. [DOI] [PubMed] [Google Scholar]
- 38.Helbling, P. M., Saulnier, D. M. & Brandli, A. W. (2000) Development (Cambridge, U.K.) 127, 269-278. [DOI] [PubMed] [Google Scholar]
- 39.Miao, H. Q., Soker, S., Feiner, L., Alonso, J. L., Raper, J. A. & Klagsbrun, M. (1999) J. Cell Biol. 146, 233-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrara, N., Gerber, H. P. & LeCouter, J. (2003) Nat. Med. 9, 669-676. [DOI] [PubMed] [Google Scholar]
- 41.Carmeliet, P. (2003) Nat. Med. 9, 653-660. [DOI] [PubMed] [Google Scholar]
- 42.LeCouter, J., Kowalski, J., Foster, J., Hass, P., Zhang, Z., Dillard-Telm, L., Frantz, G., Rangell, L., DeGuzman, L., Keller, G. A., et al. (2001) Nature 412, 877-884. [DOI] [PubMed] [Google Scholar]