Abstract
Background
Sunlight induces a wide variety of dermatoses. Their differential diagnosis is problematic not only because of similar phenotypes, but also because of confusing nomenclature and classification.
Methods
We selectively reviewed the literature of the past 20 years and describe the modern nosology of photodermatoses and their clinical features, diagnosis, and treatment. Readers should be able to provide correct and efficient diagnostic evaluation and treatment of patients with dermatoses induced by ultraviolet radiation.
Results
Photodermatoses are caused by an abnormal reaction to sunlight, usually to its ultraviolet component. They are divided into phototoxic and photoallergic reactions to known photosensitizers and idiopathic photodermatoses, in which the photosensitizer is unknown. Some types are extremely rare, such as hydroa vacciniforme (prevalence 0.34 per 100 000), while others are very common, such as polymorphic light eruption (prevalence 10% to 20%).
Conclusion
Photodermatoses are not life-threatening but can cause considerable suffering. Prevention is just as important as treatment.
Many diseases are directly or indirectly provoced or exacerbated by sunlight. Acute reactions, such as sunburn, which are induced by excessive UV radiation must be differentiated from abnormal reactions to sunlight. However, prolonged and therefore cumulative high doses of UV also prematurely age the skin and lead to damage such as skin cancer. These changes are predominantly caused by medium wavelengths (UV-B, 290–320 nm, Figure 1) and can occur in anyone with sufficiently high levels of UV exposure. Abnormal reactions to UV, however, are predominantly triggered by UV-A radiation (320–400 nm) and do not affect everyone. These diseases are considered to be true photodermatoses, the subject of this article.
Figure 1.
Spectral range of UV radiation
Photodermatoses are differentiated into primary and secondary types (Box). Primary photodermatoses are induced by photosensitizing substances. They are referred to as idiopathic primary photodermatoses if the etiology is unknown (1, 2).
Box. Photodermatoses.
Primary
-
Idiopathic
solar urticaria
polymorphous light eruption
hydroa vacciniforme
actinic prurigo
chronic actinic dermatitis
-
With known photosensitizer
phototoxic reaction
photocontact allergy
systemic photoallergy
-
Secondary
xeroderma pigmentosum
Cockayne syndrome
trichothiodystrophy
lupus erythematosus
dermatomyositis
porphyrias
pellagra
Darier’s disease
autoimmune bullous dermatoses (pemphigus, pemphigoid)
While electromagnetic radiation is the critical pathogenic factor with primary photodermatoses, secondary heliotropic diseases have another genesis altogether, although they are also induced by sunlight. Secondary photodermatoses are frequently a feature of systemic diseases such as lupus erythematosus, metabolic disorders such as porphyrias, or disorders of DNA repair such as xeroderma pigmentosum (3).
Idiopathic photodermatoses include
polymorphous light eruption
solar urticaria
hydroa vacciniforme
actinic prurigo
chronic actinic dermatitis.
Primary photodermatoses that are induced by chemical photosensitization with phototoxic or photoallergic dermatitis are differentiated from idiopathic photodermatoses.
Because confusing nomenclature and classification previously made correct diagnosis and subsequent treatment much more difficult, this review article aims to clarify modern simplified nosology of photodermatoses and their treatment. Readers should subsequently be able to make a suspected diagnosis and to plan the necessary steps for ongoing care using a directed medical history and the results of skin tests. We refer to the guidelines published by the European Dermatology Forum (www.euroderm.org/content/guidelines.htm) regarding evidence levels.
Idiopathic photodermatoses
Polymorphous light eruption
Polymorphous light eruption (PMLE)—incorrectly called sun allergy—is the most common photodermatosis with a prevalence of 10% to 20% in Central Europe, Scandinavia, and the US (2). The etiology is not yet known. The cardinal symptom is severely pruritic skin lesions. Macular, papular, papulovesicular, urticarial, multiforme- and plaque-like variants are differentiated morphologically, hence the name polymorphous (4). Usually one morphology dominates in a single individual (monomorphous).
PMLE occurs predominantly from March to June but also outside this period in tourists who have traveled to sunny regions. It can occur at any age, even in childhood. In Germany it affects women more than men (9 : 1).
Clinical manifestations
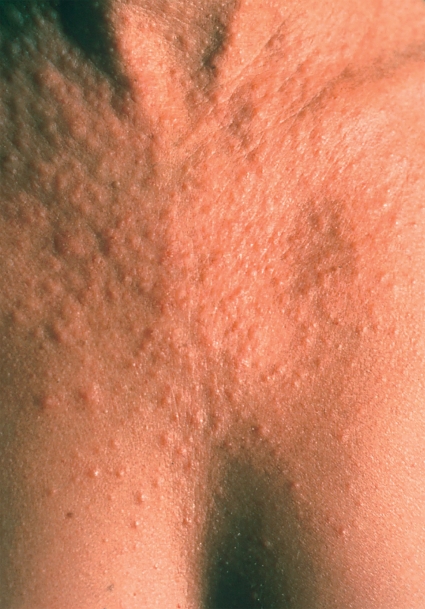
The skin lesions develop a few hours to several days after sun exposure. Initially, patchy erythema develops, accompanied by pruritus. Distinct lesions then develop (Figure 2). The upper chest, upper arms, backs of the hands, thighs, and the sides of the face are the primary localizations. The skin lesions resolve spontaneously within several days of ceasing sun exposure and do not leave behind any traces. Many patients develop tolerance over the course of the sunny period of the year, meaning that ultimately even prolonged sunbathing can be tolerated later in the season. This also explains the clustering of PMLE cases in spring or early summer but not in high summer when solar radiation is greatest.
Figure 2.
Polymorphous light eruption; small papular efflorescences 24 hours after sun exposure
Diagnosis
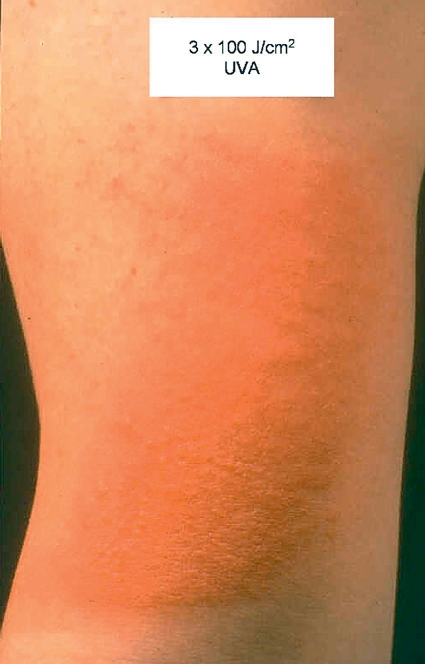
The medical history is characteristic. The skin lesions may be reproduced using experimental provocation (Figure 3) using repeated (usually over 3 days) exposure to radiation using 60–100 J/cm2 UV-A and 1.5 times the minimal erythema dose (MED) of UV-B (5). This determines the action spectrum, which in 90% of cases lies in the UV-A range.
Figure 3.
Provocation: induction of papules and vesicles after 3 exposures to UV-A radiation (100 J/cm2)
Differential diagnosis
With the papular or papulovesicular type, differential diagnosis indicates photoallergic eczema, ictus or prurigo simplex. The underlying cause of plaque-type lesions may be delayed onset solar urticaria, erythema multiforme and even lupus erythematosus (LE), particularly the tumid type. However, a latency of 1 to 3 weeks after sun exposure and a long healing time is typical for LE. A dermatological maxim states that “a patient gets PMLE on holiday but brings LE home.” Although PMLE is the most important differential diagnosis for cutaneous LE, it virtually never changes into LE, even if nonspecific positive antinuclear antibodies are present (2, 6).
Treatment
Treatment must differentiate between symptomatic treatment of manifested polymorphous light eruption and prevention. The first is simple as avoiding further sun exposure leads to rapid and spontaneous remission. It can be accelerated by external application of glucocorticoids. Antihistamines may alleviate the pruritus but their value should not be overestimated. The same applies to topical antihistamines.
Prevention is fundamentally more important. Light tolerance or hardening can be accelerated using phototherapy before the sunny period of the year. Total body radiation using UV-A and/or UV-B is suitable. Preventative radiation should only be administered under specialist medical supervision and not in a solarium in order to ensure minimal UV exposure. Topical application of broad-spectrum sunscreen is useful. This predominantly benefits UV-B-induced polymorphous light eruption. With an extremely low UV-A threshold, PMLE episodes cannot be prevented even with very potent UV-A filters. General sun protection measures such as covering with clothes and appropriate, sensible behavior are also useful. An interesting new approach to preventative external therapy involves topical application of suitable antioxidants, because pathophysiologically the inflammation reactions are most likely mediated by free radicals generated in the skin. Photochemotherapy is exceptionally effective but should, however, be reserved for extremely light-sensitive patients (7).
Solar urticaria
Solar urticaria is a rare (0.08% of all urticaria cases) but severe disorder (8). Urticarial skin lesions appear a few minutes after exposure. Anaphylactic shock may occur after whole-body exposure. The action spectrum ranges over the entire UV spectrum up to visible light. Most patients react to UV-A and visible light (5, 8).
The diagnosis is made based on the medical history and the clinical symptoms. Radiation with different wavelengths (UV-C, UV-B, UV-A, visible light) can be used to determine the action spectrum and the threshold (minimal urticaria dose, MUD) (Figure 4). In vitro pre-radiation of the patient’s plasma or serum is also an option because some patients develop a urticarial reaction at the site of injection of the irradiated plasma.
Figure 4.
Solar urticaria. Induction of erythema and wheals after radiation with UV-A
Differential diagnosis
Erythropoietic protoporphyria (diffuse swellings with petechiae rather than wheals, pain, no pruritus), urticarial phototoxic reaction after taking medication, physical urticaria (absence of UV correlation), and urticarial manifestations of polymorphous light eruption can be considered as differential diagnoses.
Treatment
Systematic treatment using antihistamines was often recommended previously but this has proven, like most other systemic medications, to be overwhelmingly inadequate because most patients have an extremely low UV threshold. Repeated solar radiation leads to light hardening that can probably be ascribed to exhaustion of the pathophysiological steps and which is used therapeutically. This phase of tolerance only lasts for two to three days, however, meaning that the radiation must be continued. The ultra-rush scheme, in which hardening is achieved using increasing daily UV-A doses applied repeatedly within a few days under inpatient conditions has, like rapid hyposensitization, proven to be effective when beginning phototherapy (9).
Photochemotherapy (PUVA, psoralene + UV-A) has become established as the method of choice for severe forms of solar urticaria because this procedure can achieve longer remissions (2 to 3 weeks) compared to radiation without psoralene (a few days). Prior to starting it is recommended to develop tolerance using repeated provocative radiation over the entire integument. PUVA treatment can then be initiated overlapping with this light hardening. After verification of the presence of a plasma factor, which is hypothetically formed by UV absorption and mediates type I reactions, treatment using plasmapheresis can achieve an improvement.
Hydroa vacciniforme
Hydroa vacciniforme is a very rare disorder (prevalence: 0.34 per 100 000) with acute onset. Numerous hemorrhagic vesicles on the face and the hands, which heal with varioliform scarring, are characteristic of the disorder. The etiopathogenesis is unknown although the most recent reports indicate that Epstein-Barr virus may play a role (10, 11) (eBox).
eBOX. Hydroa vacciniforme.
Hydroa vacciniforme usually manifests for the first time in childhood. The disorder recurs each spring. It spontaneously abates in adulthood.
Clinical manifestations
In response to the spring sun distinct inflamed reddened skin develops on the ears, nose, cheeks, fingers, backs of the hands, and the lower arms, on which blisters with serous or hemorrhagic content develop. These dry out with the formation of a blackish scab. After shedding of the scab, depressed, varioliform, often hypopigmented scars remain. In addition, hyper- and hypopigmentation are present together, resulting in a polymorphous skin presentation. There have also been severe cases accompanied by fever and a reduced general condition.
Differential diagnosis
Erythropoietic and hepatic porphyrias must be excluded by determining the level of porphyrins in the blood and urine. Further differential diagnoses include phototoxic reactions, vesiculobullous form of polymorphous light eruption, and actinic prurigo.
Treatment
A causal and effective treatment is not known. Both direct and indirect sunlight should be avoided. UV-blocking sunglasses are recommended to protect the cornea. Symptomatic treatment of the hemorrhagic crusted blisters is done topically. The skin must be covered with very high sun protection factor broad-spectrum sunscreen or completely covered with make-up or skin-colored lotion. PUVA treatment (PUVA, photochemotherapy with psoralene + UV-A) is indicated in spring before the sunny times of the year. In severe cases systemic glucocorticoids can also be used (17).
Chronic actinic dermatitis
The term chronic actinic dermatitis (CAD) now combines different medical conditions: persistent light reaction, actinic reticuloid, and photosensitive eczema. Due to the broad similarities and overlapping criteria, the term CAD is now used as an umbrella term (12). For persistent light reaction it was previously hypothetically discussed whether a photoallergy leads to chronification of the inflammatory lesions, which remain even after elimination or avoidance of the photoallergen, via persistence of the sensitizer. This could never be proven, although contact allergens and photoallergens could often be demonstrated in patients with CAD. An etiological correlation still remains unresolved, however, meaning that CAD is considered one of the idiopathic photodermatoses (13).
Clinical manifestations
CAD is characterized by chronic, usually lichenified dermatitis on sun-exposed skin areas also spreading to other areas which, although covered by clothing, are not adequately protected from the sun. The skin is inflamed and reddened, often livid red with diffuse cushion-like hardening, furrowed and scaly. The distressing pruritus leads to excoriation. The most commonly affected sites are the forehead, cheeks, ears, nape, throat and the backs of the hands and in severe cases the entire integument. Because the action spectrum can be very broad and can range from UV-B to the visible range and because the light sensitivity is often also high, the smallest quantities of light, which can also penetrate thin clothing, are sufficient to develop the chronic skin inflammation. Affected patients usually exhibit an extremely low minimal erythema dose (MED) as well as a very low UV-A threshold and are therefore severely impaired.
Differential diagnosis
CAD must be differentiated from systemically induced photoallergic reaction with constant exposure to the photosensitizer. Further differential diagnoses include aerogenic contact dermatitis, mycosis fungoides, and chronic atopic eczema.
Treatment
Avoiding the radiation that triggers the dermatitis has the highest priority. Due to the large action spectrum, the use of particularly intensive sun protection is important. In extreme cases artificial lighting at work can also contribute to continued persistence of the eczema. Shifting leisure activities to the evening and night, wearing light-blocking clothing, and full-coverage, tinted make-up preparations can also help.
PUVA treatment has become the method of choice in addition to systemic glucocorticosteroids, azathioprine, and cyclosporine A. Initiating the treatment can be very difficult due to the extreme light sensitivity. Initial doses below the eczema threshold must be selected. A combination of immunosuppressants is useful in this initial phase. A combination of cyclosporine A and PUVA should be avoided due to the risk of photocarcinogenesis (13).
Actinic prurigo
Actinic prurigo is a rare photodermatosis that develops in childhood and has a chronic-persistent course (14). Larger series have been described in England and Scandinavia although precise epidemiological data are lacking. A familial variant is present in indigenous peoples in North and Latin America (15). It is identified there as hereditary polymorphous light eruption or familial actinic prurigo. There appears to be an association with HLA-DR4. The discussion about whether this is an actual entity or a variant of PMLE is still ongoing, despite clear differences (Table).
Table. Clinical features of actinic prurigo versus polymorphous light eruption (PMLE) (from [12]).
| Feature | Actinic prurigo | PLME |
| Starts | between 2 and 9 years of age | between 9 and 29 years of age |
| Relation to UV exposure | often noted later | clear |
| Covered areas of body affected | frequent | rare |
| Scar formation | frequent | very rare |
| Pruritus | severe, persistent | transient |
| Distal one-third of nose affected | frequent | never |
| Plaques on the philtrum | frequent | never |
| Cheilitis of the lower lip | frequent | never |
| Ears affected | frequent | rare |
| Conjunctivitis | possible | never |
| HLA association | DR4/DRB1*0407 | none |
| Prophylaxis | difficult | easy |
Clinical manifestations
Pruriginous skin lesions develop on chronically sun-exposed areas of the skin such as the face, often centrofacial, the nape, the ears, the backs of the hands and the lower arms. Lesions may be found on covered skin sites as well, although these are less severe. Often exfoliative cheilitis of the lower lip and sometimes conjunctivitis are also present. Pruritus is the primary symptom. Immediately after sun exposure edematous erythema develops which slowly subsides and gradually transitions to an eczematoid phase then a pruriginous phase. Actinic prurigo persists into adulthood, with few patients showing improvement during adolescence. Lichenified erythematous plaques, cushion-like infiltrations, and nodular prurigo develop.
Differential diagnosis
Actinic prurigo must be differentiated from photo-aggravated atopic eczema, PLME, and chronic actinic dermatitis.
Treatment
Treatment is very difficult. The treatment of choice is thalidomide, the use of which must be examined in depth considering the serious adverse reactions (teratogenicity, irreversible neuropathies). Other than that, no local or systemic medication has yet been able to achieve a substantial improvement. Even light hardening using phototherapy has often had little effect on the clinical symptoms (15).
Photodermatoses with known photosensitizer
Phototoxic dermatitis
Phototoxic skin reactions are more common than photoallergic reactions. They are inflammatory skin reactions induced photochemically in exposed areas without an immunological basis. They manifest usually as dermatitis consistent with the symptoms of sunburn reaction. Phytophotodermatitis (grass dermatitis) and phototoxic reactions induced by medications such as tetracyclines are clinically significant (16).
Photosensitizing substances can be either of endogenous origin (porphyrins) or iatrogenic (medications). Systemic medications with phototoxic effects include
phenothiazines
furocoumarins
furosemide
amiodarone
tiaprofenic acid
ciprofloxacin.
UV doses that are normally tolerated without reaction may, in combination with photosensitizing substances, result in sunburn-like skin reactions. Phototoxic reactions are known after local application of furocoumarins (derived from plants, for example), acridine dyes or eosins.
Clinical manifestations
The symptoms are similar to sunburn and include acute dermatitis with reddening, edema, vesicles or blisters, and subsequently often severe pigmentation. Tetracyclines in particular induce phototoxic distal onycholysis. Phototoxic reactions after amiodarone therapy are accompanied by slate-gray, usually irreversible pigmentation. Striped, sharply delineated erythema that correspond to the trails of grass brushing on the skin are typical for grass dermatitis (Figure 5).
Figure 5.
Phototoxic dermatitis; streaked erythema after UV exposure; was cutting a bush in the garden immediately beforehand
Diagnosis
Medical history and typical findings lead to a diagnosis. If required, the photosensitizer can be verified using internal photoprovocation or a photopatch test.
Treatment
The use of all medications and cosmetics with phototoxic actions must be discontinued. Systematic use of fragrance-free sunscreen is essential. Pronounced depigmentation can be achieved using a combination of 0.1% retinoic acid, 5.0% hydroquinone and 1% hydrocortisone. Occasionally, however, persistent hyperpigmentation develops. In these cases laser therapy (Rubin laser) can help.
Photoallergic dermatitis
Photoallergic reactions occur, unlike phototoxicity, only if a specific sensitization has been acquired. They are, therefore, much more rare. Photoallergizing substances can lead to sensitization by contact or oral ingestion. Some substances are both contact allergens and photo(contact) allergens, making testing complicated. In rare cases a medication can induce a contact allergy, a photocontact allergy, and a phototoxic reaction. Important topical photoallergens include halogenated salicylanilide, fenticlor, hexachlorophene, bithionol, and in rare cases also sunscreens. A photocontact allergy persists—like a contact allergy—for a lifetime. The prevalence and incidence of photoallergic dermatitis are not precisely known. Data from large studies using the photopatch test led to the conclusion that photocontact allergies make up about 1% to 2% of all contact allergies (17).
Clinical manifestations
The acute clinical manifestations are restricted to light-exposed areas of the skin, specifically to those areas where there was contact with photoallergenic triggers. There are signs of allergic contact dermatitis with erythemas, papular vesicles, and rarely blisters. The affected skin areas differ from those parts of the body protected from light by clothing. Phototoxic reactions are defined by their extremely sharp margins (contact traces) and their rather monomorphous character (Figure 5).
The clinical symptoms shift with continued exposure to the allergen to a chronic form (chronic photoallergic contact eczema). The skin is only slightly inflamed and reddened but lichenified and scaly (Figure 6). Foci are not seen on bare skin or body parts not exposed to light, provided clothing gives adequate sun protection; however, spreading does occur. Patients complain about severe pruritus.
Figure 6.
Photoallergic dermatitis; maculopapular eczema with incipient lichenification in the UV-exposed areas
Treatment
Treatment consists of neutralization of the photoallergen. The acute or chronic clinical symptoms are treated as for eczema of allergic origin. Sun protection using both dense clothing and sunscreen (UV-A filters) is essential.
Systemic photoallergy
The clinical symptoms of systemic photoallergy are broadly similar to those of acute or chronic photoallergic contact reaction. The allergen route, for phenothiazine, sulfonamides, hydrochlorothiazide or quinidine derivatives, for example, is enteral/parenteral, however.
Diagnosis
The diagnostic procedure corresponds to that for photoallergic contact dermatitis or contact eczema. Photopatch tests are often negative for photoallergy with medications delivered by enteral or parenteral routes because a specific metabolite is actually the relevant photoallergen. Diagnosis is made using systemic photoprovocation in which a control patch is irradiated with UV-A and then the relevant medication is applied systemically. At the time of the highest plasma concentration an additional patch of skin is irradiated with UV-A and then measured after 24 and 48 hours (5, 15).
Acknowledgments
Translated from the original German by language & letters.
Footnotes
Conflict of interest statement
Professor Lehmann receives remuneration for lectures and consulting from Galderma and Jannsen-Cilag. Professor Schwarz declares that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Lehmann P. Photodermatoses—diagnosis and treatment. Dtsch Med Wochenschr. 2004;129:259–266. doi: 10.1055/s-2004-818000. [DOI] [PubMed] [Google Scholar]
- 2.Gambichler T, Al-Muhammadi R, Boms S. Immunologically mediated Photodermatoses. Am J Clin Dermatol. 2009;10:169–180. doi: 10.2165/00128071-200910030-00003. [DOI] [PubMed] [Google Scholar]
- 3.Poblete-Gutierrez P, Burgdorf WHC, Has C, Berneburg M, Frank J. Hereditäre Photodermatosen. Hautarzt. 2006;57:1067–1082. doi: 10.1007/s00105-006-1233-5. [DOI] [PubMed] [Google Scholar]
- 4.Hönigsmann H. Polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2008;24:155–161. doi: 10.1111/j.1600-0781.2008.00343.x. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann P. Diagnostic approach to photodermatoses. J Dtsch Dermatol Ges. 2006;4:965–975. doi: 10.1111/j.1610-0387.2006.06010.x. [DOI] [PubMed] [Google Scholar]
- 6.Tzaneva S, Volc-Platzer B, Kittler H, Hönigsmann H, Tanew A. Antinuclear antibodies in patients with polymorphic light eruption: a long-term follow-up study. Br J Dermatol. 2008;158:1050–1054. doi: 10.1111/j.1365-2133.2008.08500.x. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann P. Polymorphe Lichtdermatose: Was hilft wirklich? In: Plewig G, Degitz K, editors. Fortschritte der praktischen Dermatologie und Venerologie. Berlin Heidelberg: Springer-Verlag; 2001. pp. 336–339. [Google Scholar]
- 8.Botto NC, Warshaw EM. Solar urticaria. J Am Acad Dermatol. 2008;59:909–920. doi: 10.1016/j.jaad.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Beissert S, Ständer H, Schwarz T. UVA rush hardening for the treatment of solar urticaria. J Am Acad Dermatol. 2000;42:1030–1032. doi: 10.1016/s0190-9622(00)90299-8. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsuka T, Okita H, Otsuka S, Yamakage A, Yamazaki S. Hydroa vacciniforme with latent Epstein-Barr virus infection. Br J Dermatol. 2001;145:509–510. doi: 10.1046/j.1365-2133.2001.04392.x. [DOI] [PubMed] [Google Scholar]
- 11.Gupta G, Man I, Kemmet D. Hydroa vacciniforme: A clinical follow up of 17 Cases. J Am Acad Dermatol. 2000;42:208–213. doi: 10.1016/S0190-9622(00)90127-0. [DOI] [PubMed] [Google Scholar]
- 12.Milde P, Hölzle E, Neumann N, Lehmann P, Trautvetter U, Plewig G. Chronic actinic dermatitis. Concept and case examples. Hautarzt. 1991;42:617–622. [PubMed] [Google Scholar]
- 13.Hawk JL, Lim HW. Chronic actinic dermatitis. In: Lim HW, Hönigsmann H, Hawk JL, editors. Photodermatology Informa Healthcare. USA: 2007. pp. 169–183. [Google Scholar]
- 14.Lippert U, Schauder S, Neumann C. Actinic prurigo. Hautarzt. 2000;51:597–603. doi: 10.1007/s001050051178. [DOI] [PubMed] [Google Scholar]
- 15.Hojyo-Tomoka MT, Vega-Memije ME, Cortes-Franco R. Diagnosis and Treatment of actinic prurigo. Dermato Ther. 2003;16:40–44. doi: 10.1046/j.1529-8019.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 16.Schauder S. Phototoxische Reaktionen der Haut durch Medikamente. Dtsch Arztebl. 2005;102:A 2314–A 2319. [Google Scholar]
- 17.Neumann NJ, Hölzle E, Plewig G, Schwarz T, Panizzon RG, Breit R, Ruzicka T, Lehmann P. Photopatch testing: the 12-year experience of the German, Austrian, and Swiss photopatch test group. J Am Acad Dermatol. 2000;42:183–192. doi: 10.1016/s0190-9622(00)90124-5. [DOI] [PubMed] [Google Scholar]