Abstract
BACKGROUND
Peripheral FDG accumulation in a hepatic hemangioma presenting in a patient with prolonged fever is rare. Therefore, clinicians should pay close attention to patients with hepatic mass.
CASE SUMMARY
A 54-year-old woman with a 4-wk history of daily fevers was admitted to our hospital. A whole body 18F-Fluordesoxyglucose (PET-FDG) positron emission tomography/computed tomography (PET/CT) was performed to elucidate the source of the fever. However, whole body 18F-FDG PET/CT raised the suspicion of a malignant lesion because of peripheral FDG accumulation (SUVmax 3.5 g/mL) higher than that of the normal liver parenchyma (SUVmax 1.6 g/mL) surrounding a hypoactive area, and no other abnormalities were showed. Subsequently, the patient underwent liver mass resection. Histopathology showed a hepatic cavernous hemangioma with fatty infiltration around the lesion. The fever disappeared four days after surgery and the patient did not present any complications during follow-up.
CONCLUSION
Fatty infiltration in the peripheral parts of hepatic cavernous hemangioma may lead to subacute inflammation which further activate the Kupffer cells. This may cause prolonged fever and peripheral rim FDG accumulation on PET/CT.
Keywords: 18F-Fluordesoxyglucose positron emission tomography/computed tomography, Hepatocellular carcinoma, Fever, Fatty infiltration, Case report
Core Tip: Most of the hepatic cavernous hemangiomas (HCHs) are small, asymptomatic, and detected incidentally. The typical characteristics of HCHs on computed tomography or magnetic resonance imaging make their diagnosis straightforward. It has been suggested that low uptake of fluorodeoxyglucose could be useful to distinguish between benign hemangioma and malignant liver lesions. However, in the case presented here, a pathologically confirmed hepatic cavernous hemangioma showed a SUVmax (maximum standardized uptake value) in the margin of the lesion which was higher than that of the normal liver parenchyma. Therefore, clinicians should pay close attention to patients with hepatic mass.
INTRODUCTION
Cavernous hemangioma is the most common benign hepatic tumor, with a prevalence of 0.4% to 20% in the general population and is believed to arise from vascular malformations although some studies have suggested it might originate from hepatic areas of focal necrosis and regeneration[1,2]. It can occur in any age group, but is most prevalent in middle-aged women (the female to male ratio is 5:1)[3]. Hepatic cavernous hemangioma usually presents as a solitary lesion, but 2%-30% of patients may present with multiple lesions. Most of the hepatic cavernous hemangiomas are small, asymptomatic, and detected incidentally, but large ones can occasionally cause symptoms or complications such as fever, jaundice, nausea, vomiting, rupture. Giant lesions can stretch the Glissonean capsule causing pain that may influence the patient’s quality of life[4].
The typical characteristics of hepatic cavernous hemangiomas on computed tomography (CT) or magnetic resonance imaging (MRI) are progressive peripheral nodal enhancement at dynamic imaging and delayed centripetal fill-in, making their diagnosis straightforward[5]. Stable appearances on serial imaging and the absence of vascular flow on Doppler ultrasound are also helpful diagnostic cues for hepatic cavernous hemangiomas. However, atypical hemangiomas can be confused with malignant lesions such as intrahepatic cholangiocarcinoma, hepatocellular carcinoma, mixed hepatocellular-cholangiocarcinoma, and angiosarcoma[6]. Fluordesoxyglucose (FDG) uptake of hepatic cavernous hemangiomas is usually low[7]. Thus, 18F-FDG positron emission tomography/CT (PET/CT) is suggested to distinguish benign lesions from malignant lesions when CT and MRI suspect cavernous hepatic hemangioma but cannot exclude malignancy because of its larger size and degeneration[8]. Treatment depends on the size of the tumor and the symptoms. Asymptomatic patients and patients with hepatic lesion size ≤ 5 cm or with growth rate ≤ 3 mm per year do not require treatment[9]. Otherwise, therapy most often is surgical resection of the lesion, after which recurrence or growth of residual small lesions is rare[10]. A few cases were reported of orthotopic liver transplantation for large or diffuse bilateral lesions[11]. Transcatheter arterial embolization and radiation therapy are alternatives in patients unfit for surgery[12,13].
CASE PRESENTATION
Chief complaints
A 54-year-old woman with a 4-wk history of daily fevers was admitted to our hospital.
History of present illness
The patient had daily fevers (up to 39.2 °C) for 4 wk of unknown origin.
History of past illness
She was in good health and her past medical history was unremarkable.
Personal and family history
The patient denied any family history of malignancy.
Physical examination
Body temperature was 38.4 °C, blood pressure was 117/82 mmHg, heart rate was 85 beats/min, respiratory rate was 20 breaths/min and her oxygen saturation was 98%.
Laboratory examinations
Blood tests indicated an elevation in neutrophils (81.5%), C-reactive protein (118.2 mg/L), alkaline phosphatase (147.0 IU/L), and γ-glutamyl transpeptidase (59.9 U/L).
Imaging examinations
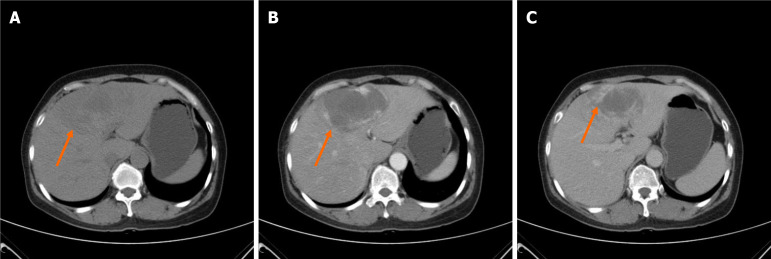
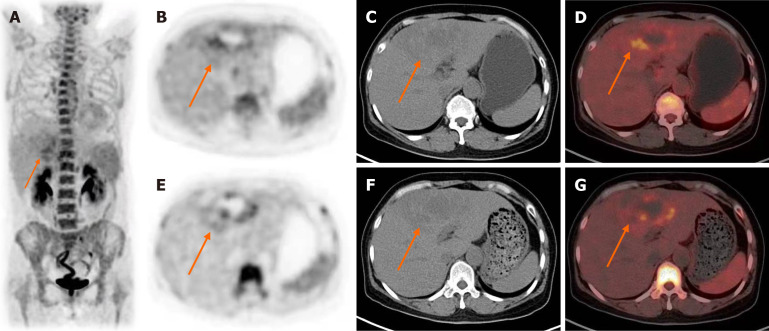
Abdominal ultrasound was performed showed a 10.0 cm × 7.0 cm heterogeneous hyperechoic mass in the left liver lobe. On dynamic contrast-enhanced CT, peripheral nodular enhancement in the arterial phase and gradual fill-in in the delayed phase were seen in the mass, which are findings typical for cavernous hemangioma (Figure 1). A whole body 18F-FDG PET/CT was performed to elucidate the source of the fever. However, whole body 18F-FDG PET/CT raised the suspicion of a malignant lesion because of peripheral FDG accumulation (SUVmax 3.5 g/mL) higher than that of the normal liver parenchyma (SUVmax 1.6 g/mL) surrounding a hypoactive area, and no other abnormalities were showed (Figure 2).
Figure 1.
Abdominal computed tomography pre-operation showed a slightly low intensity mass of 10.0 cm × 7.0 cm (orange arrow) was seen in the left liver lobe on plain computed tomography with peripheral nodular enhancement in the arterial phase and gradual fill-in in the delayed phase. A: Plain computed tomography; B: The arterial phase; C: The delayed phase.
Figure 2.
18F-Fluordesoxyglucose positron emission tomography/computed tomography pre-operation showed the liver lesion (orange arrow) with peripheral fluorodeoxyglucose accumulation (SUVmax 3.5) higher than that of the normal liver parenchyma (SUVmax 1.6) surrounds a hypoactive area. A: Whole body 18F-Fluordesoxyglucose positron emission tomography; B and E: Axial positron emission tomography; C and F: Computed tomography; D and G: Combined positron emission tomography/computed tomography slices.
FINAL DIAGNOSIS
Hepatic cavernous hemangioma.
TREATMENT
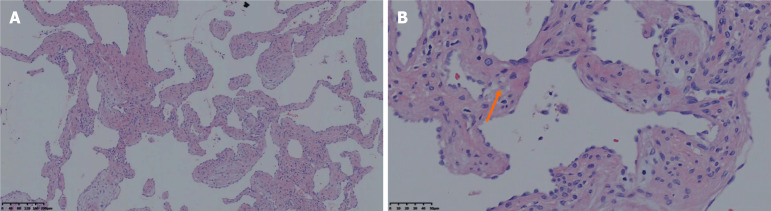
Preoperative needle biopsy was not undertaken given the risk of bleeding because the tumor was highly vascular. Subsequently, the patient underwent liver mass resection. At gross examination, the tumor was a dark red mass of 10 cm in diameter. Histopathology showed a hepatic cavernous hemangioma with fatty infiltration around the lesion (Figure 3).
Figure 3.
The result of histopathological examination. Hematoxylin and Eosin staining showed numerous dilated blood vessels adjacent to hepatocytes with fatty cell (orange arrow). A: Under low magnification (× 40); B: Under high magnification (× 100).
OUTCOME AND FOLLOW-UP
The fever disappeared four days after surgery and the patient did not present any complications during follow-up.
DISCUSSION
While it has been suggested that low uptake of FDG could be useful to distinguish between benign hemangioma and malignant liver lesions, in the case presented here, a pathologically confirmed hepatic cavernous hemangioma showed a SUVmax (maximum standardized uptake value) in the margin of the lesion which was higher than that of the normal liver parenchyma[7,8,14]. We suspect that this may result from the fatty infiltration. Uptake of FDG in focal fatty infiltration of the liver has been reported and has been attributed to activation of Kupffer cells[15]. A mouse model provided evidence that lipid accumulation in the liver leads to subacute hepatic ‘inflammation’ through nuclear factor (NF)-kappa activation and downstream cytokine production and that Kupffer cells may activated[16]. However, as the latter reference mentions, the approximately twofold activation of hepatic NF-κB is in contrast to the much greater, many-fold activation that typifies acute inflammatory reactions. Therefore, it remains speculative whether the fever in the patient described here could have been related to the uptake of FDG in the hemangioma. Fever has seldom been reported in hepatic hemangioma[17]. One study suggested that necrotic changes within the hemangioma may cause fever[18]. However, necrotic changes have not been seen in our patient. Moreover, no histologic evidence was found of infection of the hemangioma, which conceivably would constitute a differential explanation of the fever and of the FDG accumulation. Moreover, the peripheral nature of the FDG accumulation would be unexpected in case of infection and it seems unlikely that any inflammatory infiltrate would have cleared entirely. Still, this we cannot formally rule out this possibility.
CONCLUSION
Fatty infiltration in the peripheral parts of hepatic cavernous hemangioma may lead to subacute inflammation which further activate the Kupffer cells. This may cause prolonged fever and peripheral rim FDG accumulation. We observed peripheral FDG accumulation in a hepatic hemangioma presenting in a patient with prolonged fever. This probably relates to fatty infiltration at the border of the hemangioma and resulting Kupffer cell activation. Since the fever disappeared after resection of the hemangioma, this might suggest that it may have been caused by inflammation induced by the hemangioma.
Footnotes
Informed consent statement: Written informed consent has been obtained from the patient to publish this paper.
Conflict-of-interest statement: All authors declare that they have no conflicts of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Nuclear science and technology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kupeli S, Türkiye S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
Contributor Information
Yong-An Hu, Department of Nuclear Medicine, Jinhua Hospital of Zhejiang University, Jinhua Municipal Central Hospital, Jinhua 321000, Zhejiang Province, China.
Ya-Xin Guo, Department of Nuclear Medicine, Jinhua Hospital of Zhejiang University, Jinhua Municipal Central Hospital, Jinhua 321000, Zhejiang Province, China.
Qi-Feng Huang, Department of Nuclear Medicine, Jinhua Hospital of Zhejiang University, Jinhua Municipal Central Hospital, Jinhua 321000, Zhejiang Province, China. 1156225010@qq.com.
References
- 1.Ojili V, Tirumani SH, Gunabushanam G, Nagar A, Surabhi VR, Chintapalli KN, Ryan J. Abdominal Hemangiomas: A Pictorial Review of Unusual, Atypical, and Rare Types. Can Assoc Radiol J. 2013;64:18–27. doi: 10.1016/j.carj.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Brogna A, Ferrara R, Bucceri AM, Virgilio C, Scalisi G. An atypical case of hepatic cavernous hemangioma. Cases J. 2009;2:181. doi: 10.1186/1757-1626-2-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi BY, Nguyen MH. The diagnosis and management of benign hepatic tumors. J Clin Gastroenterol. 2005;39:401–412. doi: 10.1097/01.mcg.0000159226.63037.a2. [DOI] [PubMed] [Google Scholar]
- 4.Abdel Wahab M, El Nakeeb A, Ali MA, Mahdy Y, Shehta A, Abdulrazek M, El Desoky M, Abdel Wahab R. Surgical Management of Giant Hepatic Hemangioma: Single Center's Experience with 144 Patients. J Gastrointest Surg. 2018;22:849–858. doi: 10.1007/s11605-018-3696-y. [DOI] [PubMed] [Google Scholar]
- 5.Oldhafer KJ, Habbel V, Horling K, Makridis G, Wagner KC. Benign Liver Tumors. Visc Med. 2020;36:292–303. doi: 10.1159/000509145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin S, Zhang L, Li M, Cheng Q, Zheng S. Atypical hemangioma mimicking mixed hepatocellular cholangiocarcinoma: Case report. Medicine (Baltimore) 2017;96:e9192. doi: 10.1097/MD.0000000000009192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higashiyama S, Kawabe J, Hayashi T, Kurooka H, Oe A, Kotani J, Kawamura E, Shiomi S. A case of cavernous hemangioma in which malignancy was preoperatively excluded by FDG-PET. Ann Nucl Med. 2008;22:327–330. doi: 10.1007/s12149-007-0101-4. [DOI] [PubMed] [Google Scholar]
- 8.Shimada K, Nakamoto Y, Isoda H, Saito H, Arizono S, Shibata T, Togashi K. FDG PET for giant cavernous hemangioma: important clue to differentiate from a malignant vascular tumor in the liver. Clin Nucl Med. 2010;35:924–926. doi: 10.1097/RLU.0b013e3181f9de11. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Chen Z, Prasoon P, Wu H, Zeng Y. Surgical Management for Giant liver Hemangiomas Greater Than 20 cm in Size. Gut Liver. 2011;5:228–233. doi: 10.5009/gnl.2011.5.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leon M, Chavez L, Surani S. Hepatic hemangioma: What internists need to know. World J Gastroenterol. 2020;26:11–20. doi: 10.3748/wjg.v26.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prodromidou A, Machairas N, Garoufalia Z, Kostakis ID, Tsaparas P, Paspala A, Stamopoulos P, Sotiropoulos GC. Liver Transplantation for Giant Hepatic Hemangioma: A Systematic Review. Transplant Proc. 2019;51:440–442. doi: 10.1016/j.transproceed.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Hanks BA, Suhocki PV, DeLong DM, Doan PL, Liu E, Tsai AL, Burke CT, Bernard SA, O’Neil BH, Morse MA. The efficacy and tolerability of transarterial chemo-embolization (TACE) compared with transarterial embolization (TAE) for patients with unresectable hepatocellular carcinoma (HCC) J Clin Oncol. 2008;26 [Google Scholar]
- 13.Shimizu S, Mizumoto M, Okumura T, Li Y, Baba K, Murakami M, Ishida T, Nakamura M, Hiroshima Y, Iizumi T, Saito T, Numajiri H, Nakai K, Hata M, Sakurai H. Proton beam therapy for a giant hepatic hemangioma: A case report and literature review. Clin Transl Radiat Oncol. 2021;27:152–156. doi: 10.1016/j.ctro.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mergo PJ, Ros PR. Benign lesions of the liver. Radiol Clin North Am. 1998;36:319–331. doi: 10.1016/s0033-8389(05)70025-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Kim JY, Jang SJ, Chung HW, Jang KS, Paik SS, Song SY, Choi YY. F-18 FDG uptake in focal fatty infiltration of liver mimicking hepatic malignancy on PET/CT images. Clin Nucl Med. 2011;36:1146–1148. doi: 10.1097/RLU.0b013e3182335f60. [DOI] [PubMed] [Google Scholar]
- 16.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimizu C, Ariizumi S, Kogiso T, Sagawa T, Taniai M, Honda G, Egawa H, Tokushige K. Giant Hepatic Hemangioma Causing Prolonged Fever and Indicated for Resection. Intern Med. 2022;61:1849–1856. doi: 10.2169/internalmedicine.8405-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YG, Chen WF, Mai WH, Li XF, Zhou HL, Liu LJ, Li MY. Spontaneous intracapsular hemorrhage of a giant hepatic cavernous hemangioma: a rare case report and literature review. BMC Gastroenterol. 2021;21:84. doi: 10.1186/s12876-021-01666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]