Summary
Background and objectives
Up to 95% of children presenting with steroid-resistant nephrotic syndrome in early life will have a pathogenic single-gene mutation in 1 of 24 genes currently associated with this disease. Others may be affected by polymorphic variants. There is currently no accepted diagnostic algorithm for clinical genetic testing. The hypothesis was that the increasing reliability of next generation sequencing allows comprehensive one-step genetic investigation of this group and similar patient groups.
Design, setting, participants, & measurements
This study used next generation sequencing to screen 446 genes, including the 24 genes known to be associated with hereditary steroid-resistant nephrotic syndrome. The first 36 pediatric patients collected through a national United Kingdom Renal Registry were chosen with comprehensive phenotypic detail. Significant variants detected by next generation sequencing were confirmed by conventional Sanger sequencing.
Results
Analysis revealed known and novel disease-associated variations in expected genes such as NPHS1, NPHS2, and PLCe1 in 19% of patients. Phenotypically unexpected mutations were also detected in COQ2 and COL4A4 in two patients with isolated nephropathy and associated sensorineural deafness, respectively. The presence of an additional heterozygous polymorphism in WT1 in a patient with NPHS1 mutation was associated with earlier-onset disease, supporting modification of phenotype through genetic epistasis.
Conclusions
This study shows that next generation sequencing analysis of pediatric steroid-resistant nephrotic syndrome patients is accurate and revealing. This analysis should be considered part of the routine genetic workup of diseases such as childhood steroid-resistant nephrotic syndrome, where the chance of genetic mutation is high but requires sequencing of multiple genes.
Introduction
Approximately 20% of children with nephrotic syndrome will be resistant to steroids and other immunosuppression either at first presentation or subsequently. In the United Kingdom, between 350 and 400 children are affected, with 25% of these children currently on the end stage renal failure program (1). Steroid-resistant nephrotic syndrome (SRNS) is considered to represent glomerular disease resulting from a number of different etiologies leading to similar patterns of glomerular damage. The main renal histology detected is FSGS.
Positional cloning of causal genes for high-penetrance monogenic nephrotic syndromes has vastly increased our knowledge of the molecular genetic background to SRNS over the last decade. SRNS (including congenital nephrotic syndrome) displays considerable genetic heterogeneity, with 24 genes currently linked to this disorder (Table 1). In familial SRNS and SRNS presenting in the first 2 years of life, identifiable mutations are present in >95% of cases (2). This percentage decreases to around 40%–60% in children under 5 years, and in older children with sporadic disease, the incidence of genetic mutations is estimated to be 20% (2). SRNS associated with genetic mutations may progress more rapidly compared with idiopathic disease, and response to steroids or other immunosuppression drops from 40%–50% to 8% (3).
Table 1.
Genes associated with SRNS
| Gene | Protein | Disease Association |
|---|---|---|
| ACTN4 | α-Actinin 4 | Familial and sporadic SRNS (adult) |
| ALG1 | Asparagine-linked glycosylation 1 | Congenital disorder of glycosylation |
| APOL1 | Apolipoprotein L1 | Increased susceptibility to FSGS |
| CD2AP | CD2-associated protein | FSGS/SRNS |
| COL4A3 | Collagen, type IV, α3 | Alport’s disease |
| COL4A4 | Collagen, type IV, α4 | Alport’s disease |
| COL4A5 | Collagen, type IV, α5 | Alport’s disease |
| COQ2 | Coenzyme Q2 homolog | Mitochondrial disease/isolated nephropathy |
| INF2 | Inverted Formin-2 | Familial and sporadic SRNS |
| LAMB2 | Laminin, β2 | Pierson syndrome |
| LMX1B | LIM homeobox transcription factor 1β | Nail patella syndrome |
| MHY9 | Nonmuscle myosin heavy chain 9 | MYH9-related disease |
| MYO1E | Myosin IE | Familial SRNS |
| NPHS1 | Nephrin | Congenital nephrotic syndrome/SRNS |
| NPHS2 | Podocin | Congenital nephrotic syndrome/SRNS |
| PDSS2 | Prenyl (decaprenyl) diphosphate synthase, subunit 2 | Leigh syndrome |
| PLCe1 | Phospholipase C, ε1 | Congenital nephrotic syndrome/SRNS |
| PMM2 | Phosphomannomutase 2 | Congenital disorder of glycosylation |
| PTPRO | Protein tyrosine phosphatase, receptor type, O | Familial SRNS |
| SCARB2 | Scavenger receptor class B member 2 | Action myoclonus renal failure syndrome |
| SMARCAL1 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A-like protein 1 | Schimke immuno-osseous dysplasia |
| TRPC6 | Transient receptor potential cation channel, subfamily C, member 6 | Familial and sporadic SRNS (adult) |
| WT1 | Wilms tumor 1 | Sporadic SRNS (children—may be associated with abnormal genitalia) |
| ZMPSTE24 | Zinc metallopeptidase STE24 homolog | Mandibuloacral dysplasia |
Twenty-four genes associated with steroid-resistant nephrotic syndrome (SNRS) of congenital, childhood, or adult onset, familial and sporadic origin, and different syndrome association. SW1/SNF, SWItch/Sucrose Non-Fermentable; MYH9, myosin heavy chain 9, non-muscle; LIM, LIN-11, Islet-1, and MEC-3.
Although study of rare hereditary nephrotic disorders has helped our understanding of the molecular biology, the big challenges facing clinicians are how to accurately assign disease-causing roles to genetic variants detected in a specific patient, enable more accurate diagnosis/prognosis, and translate findings into concrete clinical advances that slow or abrogate disease progression.
The purpose of this study was to test the effectiveness of next generation sequencing (NGS; also known as second generation sequencing) in genes of interest in SRNS and correlate with detailed phenotypic information. The identification of causal genes has leapt forward with the advent of NGS technologies, because vastly greater amounts of DNA sequence data can now be obtained more rapidly and at less cost. However, with accumulation of very large datasets, it is also becoming increasingly apparent that correct evaluation and interpretation of any variants detected depend crucially on shared information held in open-access collections of accurate data on specific genetic disorders. The comprehensive and discriminatory phenotypic information contained within these databases can be used to curate and annotate sequence data, and at least, they indicate the degree of support for causality for a particular variant.
Our results show the obvious clinical potential for this technique, and we propose that NGS could be of equivalent quality to conventional Sanger methodology, particularly in diseases such as SRNS, where genetic heterogeneity is a defining feature.
Materials and Methods
Patients
The United Kingdom SRNS study is developing a cohort of all patients in the United Kingdom with childhood SRNS. Patients with childhood SNRS are defined as patients with congenital and familial/syndromic nephrotic syndrome as well as childhood steroid resistance (either at presentation or a later stage) with biopsy findings including minimal change, mesangial hypertrophy, and FSGS or end stage, if the clinical picture was typical of nephrotic syndrome. The following conditions were excluded: IgA and Henoch Schonlein purpura nephropathies, membranoproliferative GN and membranous nephropathy, and secondary causes, such as lupus, vasculitides, etc.
Thirty-six patient samples were drawn from the national cohort of 220 (and growing) patients. The clinical features are given in Table 2. In summary, these patients had either congenital nephrotic syndrome or childhood-onset nephrotic syndrome resistant to steroid therapy. Detailed phenotypic descriptions were logged onto the database. Familial cases are defined as patients with affected first-degree relatives. Patients were between 1 month and 16 years at onset of disease.
Table 2.
Clinical details
| Patient | Sex | Ethnicity | Age at Onset (yr or mo) | Familial (Family a, b, c, d, or e) or Sporadic | Phenotype | Length of Follow-Up (Yr) | Current CKD Stage | Time to ESRF (mo) | Disease Recurrence if Transplanted |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | NA | Birth | Fam (a) | C | 7 | 1 | n/a | n/a |
| 2 | M | In | 1 mo | S | C | 6 | 5 (HD) | 42 | n/a |
| 3 | F | W | 2 mo | S | C | 3 | 3 | n/a | n/a |
| 4 | F | NA | 3 mo | Fam (a) | C | 10 | 1 | n/a | n/a |
| 5 | M | In | 3 mo | Fam (b) | P | — | — | — | — |
| 6 | F | W | 11 mo | Fam (c) | P | 13 | 3 | n/a | n/a |
| 7 | M | Pa | 1 yr | S | P | 6 | 5 | 12 | No |
| 8 | F | W | 2 yr | S | P | 2 | 5 (PD) | 6 | n/a |
| 9 | F | W | 2 yr | Fam (c) | P | 2 | 1 | n/a | n/a |
| 10 | M | W | 2 yr | Fam (c) | P | 11 | 3 | n/a | n/a |
| 11 | F | Mix | 2 yr | S | P | 2 | 1 | n/a | n/a |
| 12 | M | In | 2 yr | S | P | 9 | 5 | 20 | Yes |
| 13 | F | W | 2 yr | S | P | 4 | 5 | 57 | n/a |
| 14 | M | W | 2 yr | S | P | 3 | 1 | n/a | n/a |
| 15 | M | W | 2 yr | S | P | 10 | 5 (Tx) | 36 | Yes |
| 16 | F | W | 4 yr | S | SYN | 6 | 5 | 22 | Yes |
| 17 | F | NA | 4 yr | S | P | 3 | 1 | n/a | n/a |
| 18 | F | W | 4 yr | S | P | 6 | 2 | n/a | n/a |
| 19 | M | NA | 5 yr | Fam (d) | P | 4 | 1 | n/a | n/a |
| 20 | F | W | 5 yr | S | P | 2 | 1 | n/a | n/a |
| 21 | M | W | 6 yr | S | P | 10 | 5 (Tx) | 24 | Yes |
| 22 | F | W | 6 yr | S | P | 3 | 5 (Tx) | 18 | Yes |
| 23 | F | In | 6 yr | Fam (e) | P | 3 | 1 | n/a | n/a |
| 24 | M | W | 7 yr | S | P | 7 | 5 (PD) | 78 | n/a |
| 25 | F | W | 8 yr | S | P | 2 | 1 | n/a | n/a |
| 26 | M | W | 9 yr | S | P | 14 | 1 | n/a | n/a |
| 27 | M | W | 10 yr | S | P | 6 | 5 (Tx) | 34 | Yes |
| 28 | F | W | 10 yr | S | P | 5 | 1 | n/a | n/a |
| 29 | M | NA | 10 yr | Fam (d) | P | 4 | 2 | n/a | n/a |
| 30 | M | NA | 11 yr | Fam (a) | P | 5 | 2 | n/a | n/a |
| 31 | M | W | 12 yr | S | SYN | 5 | 5 | 8 | No |
| 32 | F | In | 12 yr | S | P | 1 | 1 | n/a | n/a |
| 33 | F | Pa | 13 yr | S | P | 1 | 1 | n/a | n/a |
| 34 | F | W | 13 yr | S | P | 1 | 1 | n/a | n/a |
| 35 | F | W | 14 yr | S | P | 2 | 1 | n/a | n/a |
| 36 | M | W | 16 yr | S | S | 2 | 1 | n/a | n/a |
Clinical details per patient. C, congenital nephrotic syndrome; ESRF, end stage renal failure; F, female; Fam, familial; HD, hemodialysis; In, Indian subcontinent; M, male; Mix, mixed Caucasian and Afro Caribbean; NA, North African; n/a, not applicable; P, primary steroid resistance; Pa, Pakistani; PD, peritoneal dialysis; S, sporadic; SYN, syndrome with associated steroid-resistant nephrotic syndrome; Tx, transplanted; W, white.
Prior Sanger sequencing results obtained from either an in-house pilot sequencing project or commercial testing undertaken by local clinicians had revealed a total of 31 variants in NPHS1, NPHS2, and exons 8 and 9 of WT1 in nine patients; these patients were used a positive controls for the sequencing results.
Sequencing and Data Analyses
DNA was extracted using a QIAamp DNA Blood Mini Kit (Qiagen). Sample libraries were constructed using Illumina multiplexing sample preparation protocol (PE-930–1002; Illumina) with personal modifications (Supplemental Methods). A Roche NimbleGen sequence capture array, with probe capacity 12×135 K, was used to capture the exome of 446 genes inclusive of donor and acceptor sites. The genes were chosen on the basis of their known or potential involvement in nephrotic syndrome and glomerular function. The sequence library was hybridized for 70 hours, and then, the array was washed. Captured DNA was eluted and amplified. The final product (containing all 12 samples) was sequenced with 110-bp paired-end reads on Illumina GAIIx. Sequencing as well as base calling and demultiplexing were performed in the Transcriptomics Unit (University of Bristol).
Data analysis was performed using CLC Genomics Workbench v 4.9–5.0 (CLC Bio). Duplicate reads were removed, and reads were quality-trimmed (default parameters). Unique reads were then mapped against the human genome (hg19/GRCh37) with standard mapping parameters (Supplemental Table 1). Detection of single-nucleotide polymorphisms (SNPs) and short insertion/deletion polymorphisms (indels) was performed using listed parameters (Supplemental Table 2). Figure 1 is a flow diagram that shows variant analysis pathway. Any rare variants were subsequently validated by resequencing using Sanger sequencing (MWG, Germany). Splice site effect prediction was performed using Genomics Gateway (version 2.0 β5) identifying any SNPs that fell within 2 bp of an intron–exon boundary.
Figure 1.
Flow diagram showing mechanism for filtering variants to identify those variants of potential pathogenicity.
Mutation Prediction
The functional significance of all amino acid substitutions found in the captured genes was investigated in silico using MutPred and PolyPhen (4,5). MutPred is based on SIFT (http://sift.jcvi.org/) and was trained using neutral polymorphisms from Swiss-Prot together with deleterious mutations from the Human Genome Mutation Database (HGMD), thereby greatly increasing its reliability (6). It gives each variant a general score of the probability that the amino acid substitution is disease-associated/deleterious (0–1, where 0.5–0.75 is considered mildly deleterious and >0.75 is considered deleterious). All data were subsequently double checked using PolyPhen through Variant Effect Predictor in ensemble (http://useast.ensembl.org/tools.html) (7).
When segregating results according to pathogenicity, we have defined definitely pathogenic as those variants previously described as pathogenic in a patient with a similar phenotype, probably pathogenic as those variants where there is previous description of very similar variants in patient with similar phenotype, and possibly pathogenic as those variants that are rare and not previously described in patients, but prediction tools suggest high likelihood of deleterious effect on the protein.
Results
Validation of Sequencing
Of the 31 variants identified by Sanger sequencing before this study, 30 variants were also detected by NGS, suggesting 97% accuracy. Only one result, a heterozygous T to C change in NHPS1 (E588G; patient 21), was not detected initially, but review of the data analysis indicated that the variant was present but had not been called because of the stringency settings set for our analysis, specifically a low read depth. A total of seven reads was obtained for this nucleotide, with three reads showing the variation.
All 25 variants identified by NGS that were considered definitely or probably pathogenic were also subsequently confirmed by Sanger sequencing, with no false positives identified.
Sequencing Results
Four hundred forty-six genes of reported or potential functional connection with SRNS were analyzed in 36 patients from the United Kingdom cohort of SRNS in children; 96% of reads mapped to the reference sequence, and 76% of mapped reads were on target genes. Data analysis identified a total of 156,608 SNPs (mean of 4350 per patient). The average coverage was 45 reads (97% of regions had at least 1 read, 91% of regions had >5 reads, 81% of regions had >10 reads, and 62% of regions had >20 reads). Here, we report the results of the 24 genes connected with SRNS (Table 1) in detail.
A total of 86 unique variants were identified in those 24 genes. In the 24 genes, five SNPs were annotated as causing a possible splice site disruption: three SNPs are common SNPs (minor allele frequency [MAF]>1%), and two SNPs are not present in dbSNP135 (INF2 I685V and NPHS1 R586G), both causing amino acid substitution. No splice sites mutations listed in F for these genes were identified in our patient cohort.
To identify potential pathogenic variants, additional analysis focused on those variants that were novel or listed in HGMD as associated with SRNS (even if common) and also any other indels and nonsense variants. For the remaining missense variants, those variants of high risk were determined by an MAF of <1% or unknown (using 1000 Genomes population data, May 2011 release; http://www.1000genomes.org/node/506); 44 SNPs met these criteria and are listed in Table 3. However, in a Mendelian-type recessive disorder, significant minor alleles may have >1% frequency, and therefore, those variants with MAF between 1% and 5% were also identified and are listed in Supplemental Table 3.
Table 3.
Filtered variants in 24 SRNS associated genes
| Gene | Variant | Patients Affected (Heterozygote Unless Hom-Homozygous) | dbSNP Ref. No. (Build 135) | MAF If Available or ESP Genotype Count | HGMDa (Authors’ Comments) | PolyPhen | MutPred |
|---|---|---|---|---|---|---|---|
| ACTN4 | Arg310Gln | 23 | rs112545413 | A=0.0055 | FSGS (but also in controls) | Benign | 0.459 |
| APOL1 | Val369Glu | 20 | rs143845266 | ESP: AT-0.003 TT-0.997 | No | Probably damaging | 0.831 |
| APOL1 | Gly247Arg | 15 | rs146925617 | ESP: AG-0.001 GG-0.999 | No | Benign | 0.516 |
| APOL1 | Asn403fs | 1, 30 | rs143830837 | No data | No | n/a | n/a |
| CD2AP | Pro566Ser | 2 | Not in dbSNP | ESP: TC-0.0002 CC-0.9998 | No | Benign | 0.389 |
| COL4A4 | Ala715Val | 7 | rs76636743 | A=0.0014 | No | Benign | 0.282 |
| COL4A4 | Ala1558Val | 7 | Not in dbSNP | No data | No | Unknown | 0.418 |
| COL4A4 | Arg877Gln | 6 | rs150979437 | ESP: TC-0.005 CC-0.995 | No | Unknown | 0.526 |
| COL4A4 | Ser969b | 24 (hom) | rs35138315 | No data | Alport syndrome | n/a | n/a |
| COL4A4 | Gly999Glu | 23, 29 | rs13027659 | T=0.0073 | No | Unknown | 0.6 |
| COL4A4 | Met1399Leu | 17 | rs149117087 | No data | No | Unknown | 0.508 |
| COQ2 | Asn228Ser | 8 | rs121918232 | No data | Coenzyme Q10 deficiency | Probably damaging | 0.75 |
| COQ2 | Leu234fs | 8 | Not in dbSNP | No data | No | n/a | n/a |
| INF2 | Arg877Gln | 7 | rs142678449 | ESP: AA-0.0004 AG-0.0168 GG-0.9828 | No | Unknown | 0.447 |
| INF2 | Glu593Lys | 28 | Not in dbSNP | No data | No | Unknown | 0.502 |
| INF2 | Ile685Va | 19, 29 | Not in dbSNP | ESP: GA-0.0002 AA-0.9998 | No | Unknown | 0.527 |
| INF2 | Val557Gly | 19 | Not in dbSNP | ESP: GT-0.004 TT-0.996 | No | Unknown | 0.512 |
| LAMB2 | Gly1676Ile | 33 | Not in dbSNP | No data | No | Benign | 0.233 |
| LAMB2 | Arg575Gln | 28 | rs61729152 | T=0.0087 | No | Benign | 0.566 |
| MYH9 | Lys910Gln | 23 (hom) | Not in dbSNP | No data | Fechtner syndrome (but also in control) | Possibly damaging | 0.368 |
| MYH9 | Ala1072Thr | 2 | Not in dbSNP | No data | No | Benign | 0.357 |
| MYH9 | Lys1775Glu | 14 | rs145139708 | C=0.002 | No | Benign | 0.561 |
| MYH9 | Arg1936Gln | 10 | Not in dbSNP | No data | No | Possibly damaging | 0.203 |
| MYO1E | Ile531Met | 34 | rs140447165 | C =0.002 | No | Possibly damaging | 0.634 |
| NPHS1 | His1174Tyr | 21 | rs115489112 | A=0.0018 | No | Possibly damaging | 0.431 |
| NPHS1 | Asn1077Ser | 1, 4, 5, 7, 8, 9, 15, 26, 30 (21 hom) | rs4806213 | C=0.0836 | CNS (but also in control) | Possibly damaging | 0.269 |
| NPHS1 | Arg408Gln | 16, 26 | rs33950747 | T=0.0297 | CNS (but also in control) | Possibly damaging | 0.892 |
| NPHS1 | Pro264Arg | 22, 32, 35, 36 | rs34982899 | C=0.0105 | CNS (in a single patient as a compound het) | Benign | 0.77 |
| NPHS1 | Ala806Gly | 2 (hom) | Not in dbSNP | No data | No | Possibly damaging | 0.805 |
| NPHS1 | Glu117Lys | 11, 16, 18, 24, 25, 26, 35, 36 (1, 2, 4, 12, 20, 30 hom) | rs3814995 | T=0.3423 | CNS (but also in control) | Possibly damaging | 0.802 |
| NPHS1 | Arg586Gly | 1, 4, 30 (hom) | Not in dbSNP | No data | FSGS | Benign | 0.824 |
| NPHS1 | Ala916Ser | 6 | rs138173172 | ESP: AC 0.006 CC 0.994 | Reported in 1 patient with minimal change | Possibly damaging | 0.72 |
| NPHS1 | Thr294Ile | 9, 10 | rs113825926 | A=0.0037 | Reported in 1 patient with minimal change | Benign | 0.296 |
| NPHS1 | Val1084fs | 3 (hom) | Not in dbSNP | No data | CNS | n/a | n/a |
| NPHS2 | Pro20Leu | 17 | rs74315344 | A=0.0014 | Nephrotic syndrome, steroid-resistant | n/a | 0.721 |
| NPHS2 | Arg229Gln | 6, 10, 27 | rs61747728 | T=0.0178 | FSGS | Possibly damaging | 0.698 |
| NPHS2 | Ala284Val | 23 (hom) | Not in dbSNP | No data | Nephrotic syndrome, steroid-resistant | Possibly damaging | 0.945 |
| NPHS2 | Phe344fs | 6, 10 | Not in dbSNP | No data | FSGS | n/a | n/a |
| PLCE1 | Arg321b | 5 (hom) | Not in dbSNP | No data | FSGS | n/a | n/a |
| PLCE1 | Leu57Phe | 19, 29 | Not in dbSNP | No data | No | Possibly damaging | 0.106 |
| PMM2 | Glu197Ala | 13 | rs34258285 | C=0.0119 | Congenital disorder of glycosylation 1a (but polymorphism) | Benign | 0.826 |
| PTPRO | His362Arg | 34 | rs141042273 | ESP: AA-0.999 AG-0.001 | No | Benign | 0.392 |
| SMARCAL1 | Glu377Gln | 12 | rs2066518 | C=0.0603 | Schimke immuno-osseous dysplasia (report not clearly pathogenic) | Possibly damaging | 0.492 |
| WT1 | Ala100Gly | 1, 4 (hom) | Not in dbSNP | No data | No | Benign | 0.723 |
Variants of note in the exome of 24 genes associated with steroid-resistant nephrotic syndrome (SRNS) sequenced in 36 patients. Includes all variants described in Human Genome Mutation Database (HGMD) professional, all novel variants (not being described in single-nucleotide polymorphisms database [dbSNP] or the 1000 Genomes Project), and all variants with an minor allele frequency (MAF)<1% in the 1000 Genomes Project (http://www.1000genomes.org/node/506; allele frequency) or if not available, Exome Variant Server, National Heart, Lung and Blood Institute Exome Sequencing Project (ESP), Seattle, WA (http://evs.gs.washington.edu/EVS/). Where possible, MutPred and PolyPhen scores are also provided. CNS, congenital nephrotic syndrome; n/a, not available.
Professional release: December of 2011.
Nonsense.
Variants Discovered in Commonly Associated Genes (Tables 3 and 4).
Table 4.
Potentially pathogenic variants in 24 SRNS associated genes
| Gene | Variant | State | Patient | Familial/Sporadic | dbSNP Ref. No. | Population Frequency | PolyPhen | MutPred |
|---|---|---|---|---|---|---|---|---|
| Definitely pathogenic | ||||||||
| COL4A4 | Ser969a | Hom | 24 | Sporadic | rs35138315 | No data | n/a | n/a |
| NPHS1 | Arg586Gly | Hom | 1, 4, 30 | Familial | Not in dbSNP | No data | Benign | 0.824 |
| NPHS1 | Val1084fs | Hom | 3 | Sporadic | Not in dbSNP | No data | n/a | n/a |
| NPHS2 | Ala284Val | Hom | 23 | Familial | Not in dbSNP | No data | Probably damaging | 0.945 |
| PLCE1 | Arg321a | Hom | 5 | Familial | Not in dbSNP | No data | n/a | n/a |
| Probably pathogenic | No data | |||||||
| COQ2 | Asn228Ser | CH | 8 | Sporadic | rs121918232 | No data | Probably damaging | 0.75 |
| COQ2 | Leu234fs | 8 | Not in dbSNP | No data | n/a | n/a | ||
| NPHS1 | Ala806Gly | Hom | 2 | Sporadic | Not in dbSNP | No data | Possibly damaging | 0.805 |
| NPHS2 | Arg229Gln | CH | 6, 10 | Familial | rs61747728 | T=0.0178 | Possibly damaging | 0.698 |
| NPHS2 | Phe344fs | 6, 10 | Not in dbSNP | No data | n/a | n/a | ||
| Possibly pathogenic | ||||||||
| APOL1 | Val369Glu | Het | 20 | Sporadic | rs143845266 | ESP: AT-0.003 TT-0.997 | Probably damaging | 0.831 |
| MYO1E | Ile531Met | Het | 34 | Sporadic | rs140447165 | C =0.002 | Probably damaging | 0.634 |
| NPHS1 | Ala916Ser | Het | 6 | Familial | rs138173172 | ESP: AC 0.006 CC 0.994 | Probably damaging | 0.72 |
| NPHS2 | Pro20Leu | Het | 17 | Sporadic | rs74315344 | A=0.0014 | n/a | 0.721 |
| WT1 | Ala100Gly | Het & Hom | 1, 4 | Familial | Not in dbSNP | No data | Benign | 0.723 |
Variants identified by analysis of 24 nephrotic genes according to pathogenicity. Pathogenicity definition as described in the text. dbSNP, single-nucleotide polymorphism database; fs, frame shift; Hom, homozygous; Het, heterozygous; CH, compound heterozygous; n/a, not available.
Nonsense.
Ten of forty-four potentially pathogenic variants were in NPHS1. First, a homozygous non-synonymous (ns) SNP R586G, previously described in Finnish-type congenital nephrotic syndrome, was detected in three siblings (patients 1, 4, and 30) (8). Also in NPHS1, patient 3 (congenital nephrotic syndrome [CNS]) was found to be homozygous for V1084fsX1095 (c.3250insG), previously described in CNS (8). A novel homozygous nsSNP was identified in patient 2. Previous reports have described (as a pathogenic variant) a homozygous nsSNP at the same base (nt2417) but to a different nucleotide, resulting in a different amino acid change: C to A (A806D) (9). The MutPred score was 0.805 (damaging), supporting this variant as pathogenic.
An additional seven variants of note were detected in NPHS1, including P264R (previously described as a compound heterozygote in Finnish-type congenital nephrotic syndrome), which was found as a single heterozygous change in 2 of 36 patients and compound heterozygous (with a polymorphism) in 2 of 36 patients (10). Of the remaining six variants, five variants had been previously described in patients with SRNS but also in study controls or patients with mild self-resolving steroid-sensitive nephrotic syndrome, and therefore, the finding was of limited significance (9,11).
In NPHS2, patient 23 (childhood-onset SRNS) was homozygous for nsSNP A284V previously described in childhood-onset SRNS (12). Another nsSNP previously described in childhood-onset SRNS, P20L (13), was detected in 1 of 36 patients, whereas 3 of 36 patients carried a heterozygous R229Q variant in NPHS2. R229Q is thought to encode a hypomorphic allele and haploinsufficiency to predispose to late-onset SRNS (14). Interestingly, two of these patients were from a family of three affected siblings. These two patients (patients 6 and 10) were, in fact, compound heterozygous for the deletion, F344fsX347 (c.1032delT) and R229Q. This combination has been described before in a child with congenital SRNS (15). However, their sibling (patient 9) with a similar age of onset but different clinical course (steroid-resistant but calcineurin-responsive compared with siblings who are steroid- and calcineurin-resistant) carries neither. This finding has been confirmed here by Sanger sequencing and had already been determined by diagnostic screening before this study. A cousin who also has SRNS was also recently described as carrying this presumed pathogenic NPHS2 compound heterozygous combination as part of a larger cohort tested (16). Confirmatory sequencing of both parents has not been possible to date but would be particularly valuable here.
Variants Detected in Less Commonly Associated Genes.
In PLCe1, two variants of note were detected, including a homozygous nonsense mutation, R321X, in patient 5 (CNS and histology of diffuse mesangial sclerosis), which has been previously described in two siblings with early-onset SRNS (17). The second variant was a novel nsSNP, L57F, detected as a heterozygote in two siblings (patients 9 and 19; MutPred score low 0.106; benign).
Another homozygous nonsense mutation was detected in COL4A4; S969X (patient 24) was previously described in Alport’s syndrome (18). Review of the phenotypic information entered in our database for this patient indicated bilateral sensorineural hearing loss as well as SRNS, making a diagnosis of Alport’s syndrome complicated by FSGS rather than isolated renal disease more likely.
Additional analysis revealed that patient 11 has three heterozygous variants in COL4A4, each of which occurs with a MAF<2%. Variant prediction scores are not available from PolyPhen and low from MutPred. However, these variants can be neither dismissed as irrelevant or described as pathogenic. It is pertinent that this patient does not have sensorineural hearing loss and therefore, is not typical of Alport’s syndrome, and original histology was not typical of thin basement membrane disease. In liaison with the local clinician, we have advised screening family members for proteinuria and will perform parental DNA sequencing.
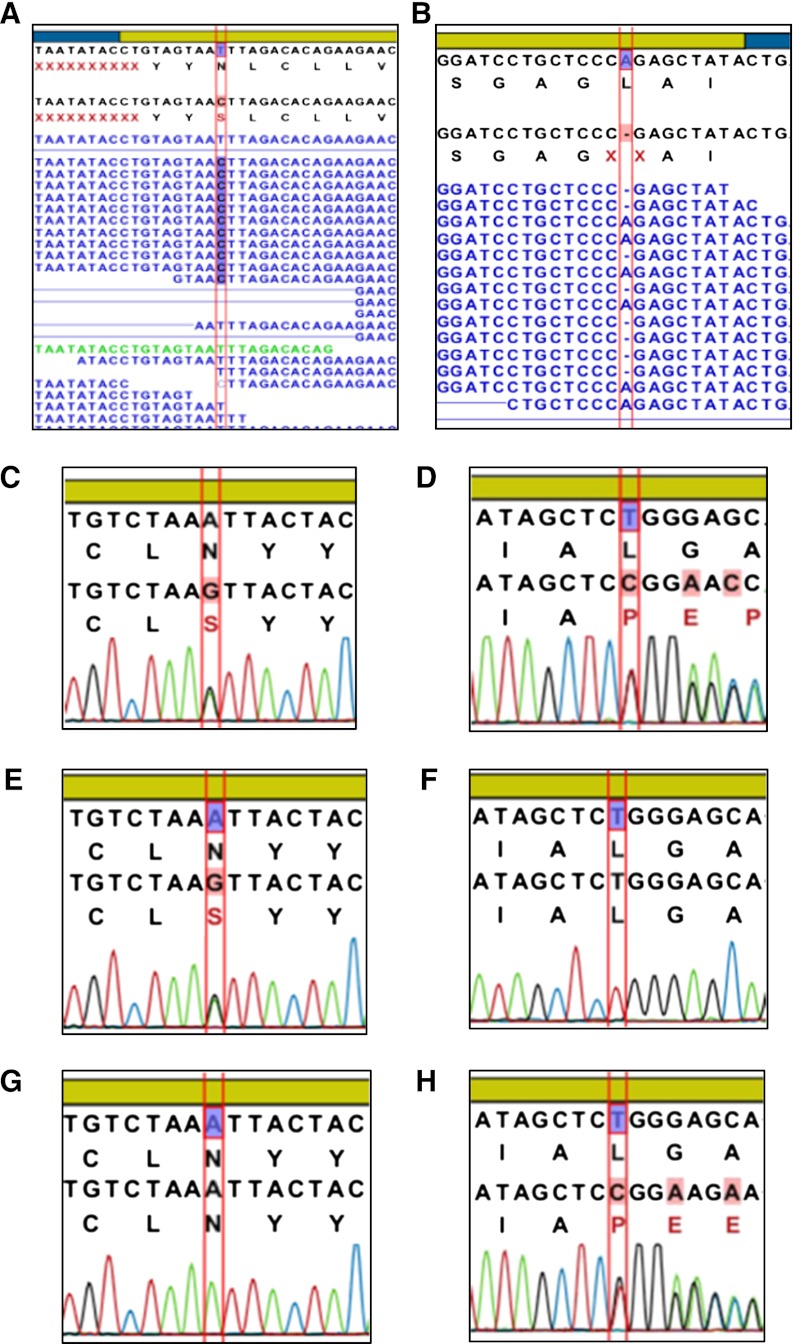
Patient 8 was compound heterozygous for a novel deletion in COQ2, L234fsX247 (c.701delT), and a heterozygous nsSNP, N228S, previously described in a single case report of a patient with an apparently isolated severe SRNS presenting at age 18 months and deteriorating rapidly into end stage renal failure within weeks. Magnetic resonance imaging and neurologic examination remained normal at follow-up of 8 months on COQ10 supplementation (19). In the present study, patient 8 developed severe nephrosis and acute renal failure resistant to therapy at age 2 years before a rapid decline in renal function to end stage within 6 months. She remains neurologically normal, with no sensorineural hearing loss. The patient’s mother is heterozygous for the point mutation, and the father is heterozygous for the frame shift in COQ2 (Figure 2).
Figure 2.
Comparison of NGS and Sanger sequencing data acquisition Sequencing results for patient 8 and parents in gene COQ2 at nucleotide position c.683A>G (N228S; A, C, E, and G) and nucleotide position c.701delT (L234fsX247; B, D, F, and H). Next generation sequencing showed a heterozygous ns single-nucleotide polymorphism (SNP; A) and a heterozygous deletion (B) in patient 8. Both the non-synonymous (ns) SNP (C) and the deletion (D) were confirmed by Sanger methodology. The mother of the patient was confirmed to be a heterozygous carrier of the nsSNP (E) but not the deletion (F), whereas father did not carry the nsSNP (G) but was found to be heterozygous for the deletion (H).
Novel Potential Variants in Less Commonly Associated Genes.
Of the 25 remaining variants of note, four variants are ns SNPs in INF2 (three variants were novel, scoring 0.502–0.527, or possibly damaging). Interestingly, most of the disease-associated mutations described so far for INF2 have been found within the N-terminal regulatory diaphanous inhibitory domain (20). However, the variants identified here are in the Formin homology 2 domain. The Formin homology 2 domain is responsible for enhancing both actin filament nucleation and polymerization, and therefore, perturbation of this region could be expected to potentially have deleterious effects on protein function, impacting on the actin cytoskeleton (21).
Two more heterozygote nsSNPs were scored by MutPred to be probably damaging: APOL1 V369Q and PMM2 E197A. The latter has previously been described as a polymorphism (22). Four different nsSNPs were predicted by Polyphen to be probably damaging: NPHS1 H1174Y (heterozygote in patient 21), MYO1E I531M (heterozygote in patient 34), MYH9 K910G (homozygous in patient 23; previously described in a patient with Fechtner syndrome but also in control) (23), and SMARCAL1 E377Q (heterozygous in patient 12; previously described; probable polymorphism) (24).
Variants Involved in Potential Genetic Epistasis.
Interestingly, a novel WT1 SNP in exon 1 resulting in A100G (MutPred score 0.72; potentially harmful) was also detected in patients 1 and 4 but not patient 30. Patients 1 and 4 developed more aggressive disease than patient 30, who only carried R586G, supporting genetic epistasis between NPHS1 and WT1, which has previously been reported (25). Because WT1 exon 1 SNPs have previously been associated with renal disease (26,27) and no other significant changes were detected in the other 22 SRNS-associated genes, it may be that this novel variant influenced phenotype.
Discussion
This study describes the use of targeted enrichment and NGS to screen 24 genes associated with SRNS. Patients were identified nationally using a novel rare renal disease registry (www.renalradar.org) and recruited to the United Kingdom SRNS in Childhood study. As a pilot, DNA from the first 36 patients was sequenced. These samples were the first samples received, although local clinician bias as to which patients were recruited first could not be ruled out, and it is possible that the more severe or frequently reviewed patients were recruited earlier to the cohort.
In this study, 70% of patients with familial disease and 15% of sporadic cases had a definitely or probably pathogenic variant identified, and in keeping with previous reports, age of onset correlated with risk of an identifiable pathogenic variant, with a positive finding in 86% of those patients presenting before 2 years of age (Table 5). In fact, 100% of those patients presenting before 2 years who had familial disease had an identifiable mutation, although the numbers in this pilot study were small. Interestingly, sequencing this number of genes enabled identification of mutations that would rarely be considered. This finding confirms that phenotype may not easily predict genotype and that screening all known genes is clinically relevant and may provide therapeutic options.
Table 5.
Frequency of definitely or probably pathogenic variants according to age
| Age at Onset (yr) | Total | Familial | Sporadic | Variant Identified | Definite or Probable Genetic Cause (%) | |
|---|---|---|---|---|---|---|
| Definitely Pathogenic | Probably Pathogenic | |||||
| <2 | 7 | 4 | 3 | 4 | 2 | 86 |
| 2–5 | 11 | 2 | 9 | 0 | 2 | 18 |
| >5 | 18 | 4 | 14 | 3 | 0 | 17 |
SRNS is known to be a disease of particular genetic heterogeneity, with the list of causative genes growing constantly (28–30). Although clinical advances have been rather limited, the benefit of this expansion in knowledge of the pathogenetic origin of disease is significant. It is accepted that accurate early identification of known pathogenic variants will modify therapeutic approach, often avoiding unnecessary immunosuppression and allowing treatment of rare conditions, such as COQ10 biosynthetic pathway disorders. In a disease where recurrence post-transplant is high but almost unknown in those patients with a genetic origin, transplantation and particularly, live related transplantation may be explored as therapeutic options earlier.
It is apparent that this condition, like others with multiple genes involved, lends itself to a clinical diagnostic service screening several genes at one time using NGS technology. The savings and efficiency of the process compared with Sanger sequencing may be obvious, but to replace or complement Sanger technique, it would need to be considered equally as accurate. This report shows that the accuracy, based on verification in 56 separate variants detected, is now approaching levels that would make it possible to develop the technology for clinical testing. It is interesting to note that, in the United Kingdom, with diagnostic laboratories already offering services using NGS (31), the Clinical Molecular Genetics Society is working to national best-practice guidelines for the use of NGS.
This report also highlights the difficulty in interpretation of results. Identification of definite mutations is clearly useful in a clinical context, but the remaining results require careful analysis. In this report, we have listed some variants as probably pathogenic (for instance, a novel homozygous variant in NPHS1); however, a previous report describes a variant at the same codon resulting in a different amino acid change in a patient with a similar phenotype or a compound heterozygous in COQ2, where one variant is previously described as pathogenic and the other is a novel indel, resulting in significant disruption of the protein. Instinctively, these variants would seem to be pathogenic, and we would advocate reporting as such with caution.
For other variants, however, where they are rare but not previously described, the impact on patient management is less tangible, except that by reporting these types of variants by our group and others with clear genotype phenotype description, a clearer picture may be developed. With the inevitable expansion of NGS data in this disease, we propose a publicly accessible combined disease-specific database of variants in nephrotic genes with phenotypic data available as the most efficient way to define genetic pathogenicity.
An additional issue to consider is variant frequency in patients of certain ethnic origins. In the open-access databases used here, certain populations are not well described (for instance, those populations from India, Pakistan, and North Africa). In fact, using data from whole-genome databases (compared with whole-exome databases) as controls does have the disadvantage that coverage is often not high; therefore, rare variants may be missed, or allele frequencies may be underestimated. In the near future, population genetics will improve the databases of different ethnic groups.
One might also argue about what constitutes rare in a disease that, if genetic in origin, is usually autosomal-recessive. Using a cutoff MAF of 1% may be too stringent; in this study, other interesting variants were found occurring at >1% but generally below 3%.
The use of HGMD as a filtering tool is difficult. Although it is very useful to have a list of all variants associated with the disease described in the literature, in-depth analysis of the individual references revealed many were nonsignificant polymorphisms.
A particular strength of this study is the use of a new comprehensively phenotyped national cohort of pediatric SRNS patients, ensuring the most appropriate inclusion criteria for testing. The results that we present are comparable with gold standard Sanger sequencing and highly informative.
We aim to propose, based on these data, that simultaneous NGS sequencing should be considered for genetic management of all patients with SRNS.
Disclosures
None.
Acknowledgments
We are indebted to the patients and families for participating in this study and the clinical teams involved in recruitment and sample collection, including Emma Rigby, Liz Reus, Majorie Allen, Malcolm Lewis, Nick Plant, Mohan Shenoy, Rachel Lennon, Kate Baker, Kate Cotter, Kayleigh Aston, Liang Qiao, Pauline Jones, David Hughes, Hannah Leyland, Wendy Cook, Natalie Fineman, Daljit Hothi, Ambrose Gullett, Kjell Tullus, Robert Kleta, and Detlef Bockenhauer.
This study was supported by Kids Kidney Research, Kidney Research UK, North Bristol National Health Service Trust, and Nephrotic Syndrome Trust (NeST).
Footnotes
H.J.M. and A.B. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07200712/-/DCSupplemental.
References
- 1.Sinha MD, Castledine C, van Schalkwyk D, Hussain F, Lewis M, Inward C: UK Renal Registry 13th Annual Report (December 2010): Chapter 5: Demography of the UK paediatric renal replacement therapy population in 2009. Nephron Clin Pract 119[Suppl 2]: c97–c106, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Rood IM, Deegens JK, Wetzels JF: Genetic causes of focal segmental glomerulosclerosis: Implications for clinical practice. Nephrol Dial Transplant 27: 882–890, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Büscher AK, Kranz B, Büscher R, Hildebrandt F, Dworniczak B, Pennekamp P, Kuwertz-Bröking E, Wingen AM, John U, Kemper M, Monnens L, Hoyer PF, Weber S, Konrad M: Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 5: 2075–2084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B, Krishnan VG, Mort ME, Xin F, Kamati KK, Cooper DN, Mooney SD, Radivojac P: Automated inference of molecular mechanisms of disease from amino acid substitutions. Bioinformatics 25: 2744–2750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramensky V, Bork P, Sunyaev S: Human non-synonymous SNPs: Server and survey. Nucleic Acids Res 30: 3894–3900, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thusberg J, Olatubosun A, Vihinen M: Performance of mutation pathogenicity prediction methods on missense variants. Hum Mutat 32: 358–368, 2011 [DOI] [PubMed] [Google Scholar]
- 7.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F: Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 26: 2069–2070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoeb DS, Chernin G, Heeringa SF, Matejas V, Held S, Vega-Warner V, Bockenhauer D, Vlangos CN, Moorani KN, Neuhaus TJ, Kari JA, MacDonald J, Saisawat P, Ashraf S, Ovunc B, Zenker M, Hildebrandt F, Gesselschaft für Paediatrische Nephrologie (GPN) Study Group : Nineteen novel NPHS1 mutations in a worldwide cohort of patients with congenital nephrotic syndrome (CNS). Nephrol Dial Transplant 25: 2970–2976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenkkeri U, Männikkö M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac C K, Kashtan CE, Homberg C, Olsen A, Kestilä M, Tryggvason K: Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 64: 51–61, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koziell A, Grech V, Hussain S, Lee G, Lenkkeri U, Tryggvason K, Scambler P: Genotype/phenotype correlations of NPHS1 and NPHS2 mutations in nephrotic syndrome advocate a functional inter-relationship in glomerular filtration. Hum Mol Genet 11: 379–388, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Lahdenkari AT, Kestilä M, Holmberg C, Koskimies O, Jalanko H: Nephrin gene (NPHS1) in patients with minimal change nephrotic syndrome (MCNS). Kidney Int 65: 1856–1863, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Karle SM, Uetz B, Ronner V, Glaeser L, Hildebrandt F, Fuchshuber A: Novel mutations in NPHS2 detected in both familial and sporadic steroid-resistant nephrotic syndrome. J Am Soc Nephrol 13: 388–393, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Machuca E, Hummel A, Nevo F, Dantal J, Martinez F, Al-Sabban E, Baudouin V, Abel L, Grünfeld JP, Antignac C: Clinical and epidemiological assessment of steroid-resistant nephrotic syndrome associated with the NPHS2 R229Q variant. Kidney Int 75: 727–735, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, Hasselbacher K, Hangan D, Ozaltin F, Zenker M, Hildebrandt F, Arbeitsgemeinschaft für Paediatrische Nephrologie Study Group : Nephrotic syndrome in the first year of life: Two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119: e907–e919, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Balasz-Chmielewska IBH, Drozdz D, Gacka E, Firszt-Adamczyk A, Jarmolinski T, Ksiazek J, Kuzma-Mroczkowska E, Medynska A, Silska M, Szczepanska M, Tkaczyk M, Wasilewska A, Zurowska A: Early onset and intermediate progression of steroid-resistant nephrotic syndrome associated with non-neutral p.R299Q polymorphism and novel c.1032delT podocin mutation. Pediatr Nephrol 27: 1722–1723A, 2012 [Google Scholar]
- 17.Boyer O, Benoit G, Gribouval O, Nevo F, Pawtowski A, Bilge I, Bircan Z, Deschênes G, Guay-Woodford LM, Hall M, Macher MA, Soulami K, Stefanidis CJ, Weiss R, Loirat C, Gubler MC, Antignac C: Mutational analysis of the PLCE1 gene in steroid resistant nephrotic syndrome. J Med Genet 47: 445–452, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Dagher H, Yan Wang Y, Fassett R, Savige J: Three novel COL4A4 mutations resulting in stop codons and their clinical effects in autosomal recessive Alport syndrome. Hum Mutat 20: 321–322, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, Ghiggeri GM, Murer L, Barisoni L, Pastore A, Muda AO, Valente ML, Bertini E, Emma F: COQ2 nephropathy: A newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol 18: 2773–2780, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Boyer O, Benoit G, Gribouval O, Nevo F, Tête MJ, Dantal J, Gilbert-Dussardier B, Touchard G, Karras A, Presne C, Grunfeld JP, Legendre C, Joly D, Rieu P, Mohsin N, Hannedouche T, Moal V, Gubler MC, Broutin I, Mollet G, Antignac C: Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 239–245, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chhabra ES, Higgs HN: INF2 is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J Biol Chem 281: 26754–26767, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Vega AI, Pérez-Cerdá C, Abia D, Gámez A, Briones P, Artuch R, Desviat LR, Ugarte M, Pérez B: Expression analysis revealing destabilizing mutations in phosphomannomutase 2 deficiency (PMM2-CDG): Expression analysis of PMM2-CDG mutations. J Inherit Metab Dis 34: 929–939, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Seri M, Pecci A, Di Bari F, Cusano R, Savino M, Panza E, Nigro A, Noris P, Gangarossa S, Rocca B, Gresele P, Bizzaro N, Malatesta P, Koivisto PA, Longo I, Musso R, Pecoraro C, Iolascon A, Magrini U, Rodriguez Soriano J, Renieri A, Ghiggeri GM, Ravazzolo R, Balduini CL, Savoia A: MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore) 82: 203–215, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Clewing JM, Fryssira H, Goodman D, Smithson SF, Sloan EA, Lou S, Huang Y, Choi K, Lücke T, Alpay H, André JL, Asakura Y, Biebuyck-Gouge N, Bogdanovic R, Bonneau D, Cancrini C, Cochat P, Cockfield S, Collard L, Cordeiro I, Cormier-Daire V, Cransberg K, Cutka K, Deschenes G, Ehrich JH, Fründ S, Georgaki H, Guillen-Navarro E, Hinkelmann B, Kanariou M, Kasap B, Kilic SS, Lama G, Lamfers P, Loirat C, Majore S, Milford D, Morin D, Ozdemir N, Pontz BF, Proesmans W, Psoni S, Reichenbach H, Reif S, Rusu C, Saraiva JM, Sakallioglu O, Schmidt B, Shoemaker L, Sigaudy S, Smith G, Sotsiou F, Stajic N, Stein A, Stray-Pedersen A, Taha D, Taque S, Tizard J, Tsimaratos M, Wong NA, Boerkoel CF: Schimke immunoosseous dysplasia: Suggestions of genetic diversity. Hum Mutat 28: 273–283, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Löwik M, Levtchenko E, Westra D, Groenen P, Steenbergen E, Weening J, Lilien M, Monnens L, van den Heuvel L: Bigenic heterozygosity and the development of steroid-resistant focal segmental glomerulosclerosis. Nephrol Dial Transplant 23: 3146–3151, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Regev M, Kirk R, Mashevich M, Bistritzer Z, Reish O: Vertical transmission of a mutation in exon 1 of the WT1 gene: Lessons for genetic counseling. Am J Med Genet A 146A: 2332–2336, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Little S, Hanks S, King-Underwood L, Picton S, Cullinane C, Rapley E, Rahman N, Pritchard-Jones K: A WT1 exon 1 mutation in a child diagnosed with Denys-Drash syndrome. Pediatr Nephrol 20: 81–85, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, Antignac C, Pollak M, Kopp JB, Winn MP, Shaw AS: Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest 121: 4127–4137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, Buelli S, Tomasoni S, Piras R, Krendel M, Bettoni S, Morigi M, Delledonne M, Pecoraro C, Abbate I, Capobianchi MR, Hildebrandt F, Otto E, Schaefer F, Macciardi F, Ozaltin F, Emre S, Ibsirlioglu T, Benigni A, Remuzzi G, Noris M, PodoNet Consortium : MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med 365: 295–306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozaltin F, Ibsirlioglu T, Taskiran EZ, Baydar DE, Kaymaz F, Buyukcelik M, Kilic BD, Balat A, Iatropoulos P, Asan E, Akarsu NA, Schaefer F, Yilmaz E, Bakkaloglu A, PodoNet Consortium : Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am J Hum Genet 89: 139–147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan JE, Carr IM, Sheridan E, Chu CE, Hayward B, Camm N, Lindsay HA, Mattocks CJ, Markham AF, Bonthron DT, Taylor GR: Genetic diagnosis of familial breast cancer using clonal sequencing. Hum Mutat 31: 484–491, 2010 [DOI] [PubMed] [Google Scholar]